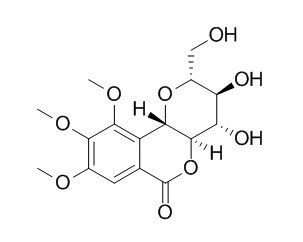
Di-O-methylbergenin
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem.2024, 436:137768.
Life (Basel).2021, 11(7):616.
Cell J.2024, 26(8):496-504.
Food Chem X.2024, 24:101909.
Pharmacol Rep.2017, 69(6):1224-1231
Front Pharmacol.2025, 16:1611342.
Molecules.2018, 23(9):E2121
Biofactors.2018, 44(2):168-179
Dermatologica Sinica2024, 42(1):p19-30.
Biomed Pharmacother.2022, 145:112410.
Related and Featured Products
massachusetts institute of technology, 2003.
Toward the automated synthesis of carbohydrates and glycosaminoglycans.[Reference:
WebLink]
Glycosaminoglycans are crucial components of the extracellular matrix and cell surface and access to define sequences is required to fully appreciate their role in biology. As is the case with most complex polysaccharides, the chemical synthesis of glycosaminoglycan structures is challenging.
METHODS AND RESULTS:
This thesis describes efforts towards the construction of oligosaccharides with the ultimate goal of the automated synthesis of glycosaminoglycans. The use of glycosyl phosphates of glucosamine and glucuronic acid as competent glycosylating agents is illustrated. Further expansion of the glycosyl phosphates methodology to the construction of C-aryl and C-alkyl glycosides and resulted in the synthesis of the natural product 8,1 0-Di-O-methylbergenin. The automated solid-phase synthesis of two complex oligosaccharides is described. The complex-type high mannose trisaccharide and the proteoglycan linkage region tetrasaccharide were synthesized in a fully automated fashion. The efforts towards the synthesis of two glycosaminoglycans - hyaluronic acid and chondroitin sulfate - are discussed.
CONCLUSIONS:
The work focuses on the discovery of a glycosylating agent suitable for the automated solid-phase synthesis. The synthesis of glucuronic acid from glucose is described using an oxidation procedure not previously explored in oligosaccharide construction.