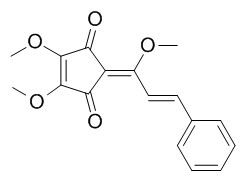
Methyllinderone
Methyllinderone is a human chymase inhibitor, it has antioxidant property, it also shows moderate to weak antifungal activities against various pathogenic fungi. Methyllinderone can inhibit farnesyl protein transferase with IC50 value of 55.3+/-4.1microM and selectively inhibit the growth of H-ras-transformed rat-2 cell lines with a GI50 value of 0.3 microM. Methyllinderone shows significant cytotoxicity against mouse melanoma (B16-F10), human acetabulum fibrosarcoma (HT1080), and choronic myelogenous leukemia (K562) cancer cell lines with ED(50) values of 2.2, 2.5, 8.3 mug/ml, respectively.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Research Square2024, 4805471.
J Agric Food Chem.2015, 63(44):9869-78
Food Res Int.2020, 133:109130.
Molecules.2024, 29(24):5983.
Front Immunol.2017, 8:1542
Food Chem.2019, 279:80-87
Food Addit Contam Part A Chem Anal Control Expo Risk Assess.2020, 37(9):1437-1448.
Int J Pharmacol2020, 16:1-9
Appl. Sci. 2021, 11(1),14.
Evid Based Complement Alternat Med.2020, 2020:2584783.
Related and Featured Products
Nat. Prod. Sci., 2002, 8(3):100-2.
Cytotoxicity of Lignans from Lindera erytheroca.[Reference:
WebLink]
METHODS AND RESULTS:
Three lignans were isolated from a methanol extract of Lindera erytherocarpa Makino (Lauraceae) are evaluated in vitro cytotoxicity using three cancer cell line assay. The compounds were identified as Methyllinderone (1), linderone (2), and kanakugiol (3) by spectroscopic methods.
CONCLUSIONS:
Amongst the compounds, Methyllinderone (1) showed significant cytotoxicity against mouse melanoma (B16-FlO), human acetabulum fibrosarcoma (HT1080), and choronic myelogenous leukemia (K562) cancer cell lines with values of 2.2, 2.5, 8.3 , respectively.
Planta Med. 2007 Jun;73(7):679-82.
Inhibition of chitin synthase 2 and antifungal activity of lignans from the stem bark of Lindera erythrocarpa.[Pubmed:
17538872]
METHODS AND RESULTS:
Potent chitin synthase 2 inhibitors, Methyllinderone (1), linderone (2) and kanakugiol (3) were isolated from the stem bark of L. erythrocarpa Makino (Lauraceae). These compounds inhibited chitin synthase 2 with IC(50) values of 23.3, 21.4 and 23.8 microg/mL, respectively. Methyllinderone (1) and linderone (2) exhibited no inhibitory activities for chitin synthases 1 and 3 from S. cerevisiae, and chitin synthase 1 from Candida albicans up to the concentration of 280 microg/mL, while kanakugiol (3) exhibited very weak activity against chitin synthase 1 of C. albicans with an IC(50) of 160 microg/mL. All of the compounds showed moderate to weak antifungal activities against various pathogenic fungi (MIC: 8 - >128 microg/mL) including Cryptococcus neoformans, Aspergillus fumigatus, and Colletotrichum lagenarium.
CONCLUSIONS:
The results indicate that these compounds are specific inhibitors of chitin synthase 2 and can potentially serve as antifungal agents.
Bioorg Med Chem. 2005 Nov 15;13(22):6182-7.
Cyclopentenediones, inhibitors of farnesyl protein transferase and anti-tumor compounds, isolated from the fruit of Lindera erythrocarpa Makino.[Pubmed:
16055336]
METHODS AND RESULTS:
Four cyclopentenediones, farnesyl protein transferase inhibitors, and anti-tumor compounds were isolated from the methanolic extract of the fruits of Lindera erythrocarpa Makino (Lauraceae). The structure of the compounds was determined by spectral data including NMR and mass spectrometry, and cyclopentenediones such as Methyllinderone (1), methyllucidone (2), lucidone (3), and linderone (4) were identified by comparing their reported spectral data with that of the literature values. Compounds 1-4 inhibited farnesyl protein transferase with IC50 value of 55.3+/-4.1, 42+/-1.9, 103+/-5.1, and 40+/-3.5 microM, respectively.
CONCLUSIONS:
Isolated compounds also inhibited the growth of various human cancer cell lines in a dose-dependent manner. Especially, Methyllinderone and methyllucidone selectively inhibited the growth of H-ras-transformed rat-2 cell lines in comparison with normal rat-2 cells with a GI50 value of 0.3 and 0.85 microM, respectively.
Contrib Nephrol. 1987;56:53-9.
The importance of the renal interstitium for kidney function.[Reference:
WebLink]
Total synthesis of human chymase inhibitor Methyllinderone has been achieved in only four steps with an overall yield of 21% from dimethyl squarate.
METHODS AND RESULTS:
We developed an efficient synthetic method for obtaining Methyllinderone derivatives and found the active compound. In addition, we propose the inhibition mechanism of the active compound against human chymase using calculations.
Mol. Phys., 2015, 113(7):683-97.
Conformational, electronic and antioxidant properties of lucidone, linderone and methyllinderone: DFT, QTAIM and NBO studies.[Reference:
WebLink]
Theoretical studies on lucidone, linderone and Methyllinderone were performed to investigate factors that contribute to structural stability and to elucidate the antioxidant properties and mechanisms.
METHODS AND RESULTS:
The study was performed in different media utilising the density functional theory with different functionals and the 6-311+ G(d,p) basis set. The antioxidant activity has been considered through the electron transfer and metal chelation mechanisms. The results show that the stability of the tautomers and conformers is due to the presence of several intramolecular hydrogen bonds.
The ionisation potential values suggest that the antiradical activity increases with the increase in the number of OCH3 groups substituted on the cyclopentene-1,3-dione ring.
In vacuo, the spin density of the Fe(II) cation upon ligand coordination decreases to 3.0−3.5, whereas the ligand spin density approaches 1, indicating that it is oxidised to a radical cation.
CONCLUSIONS:
The metal ion affinity (MIA) is influenced by the position and number of OCH3 substituted on the acylcyclopentene-1,3-dione ring.
A very favourable MIA, in vacuo, is obtained when Fe(II) is chelated between the sp2 O and sp3 O atoms. An estimation of MIA in an aqueous solution shows a remarkable decrease with respect to the results in vacuo.