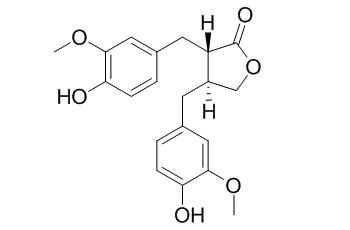
(+)-Matairesinol
(+)-Matairesinol exhibits immunomodulatory activity; it also shows inhibition of the discoloration of yellowtail dark muscle.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Insect Sci.2020, 20(5):18.
Applied Biological Chemistry2022, 71:s13765-022-00743-5.
Food Chem.2020, 332:127412
Int J Mol Sci.2022, 23(15):8687.
Food Funct.2022, D1FO03838A.
Bioorg Chem.2024, 145:107182.
J Nat Prod.2022, doi: 10.1021
Biomolecules.2024, 14(5):589.
Front Pharmacol.2021, 12:615157.
J Sep Sci.2022, 45(18):3556-3566.
Related and Featured Products
Nat Prod Commun. 2014 Jan;9(1):79-82.
Antiausterity activity of arctigenin enantiomers: importance of (2R,3R)-absolute configuration[Pubmed:
24660468]
METHODS AND RESULTS:
From a MeOH extract of powdered roots of Wikstroemia indica, six dibenzyl-gamma-butyrolactone-type lignans with (2S,3S)-absolute configuration [(+)-arctigenin (1), (+)-Matairesinol (2), (+)-trachelogenin (3), (+)-nortrachelogenin (4), (+)-hinokinin (5), and (+)-kusunokinin (6)] were isolated, whereas three dibenzyl-gamma-butyrolactone-type lignans with (2R,3R)-absolute configuration [(-)-arctigenin (1*), (-)-matairesinol (2*), (-)-trachelogenin (3*)] were isolated from Trachelospermum asiaticum. The in vitro preferential cytotoxic activity of the nine compounds was evaluated against human pancreatic PANC-1 cancer cells in nutrient-deprived medium (NDM), but none of the six lignans (1-6) with (2S,3S)-absolute configuration showed preferential cytotoxicity. On the other hand, three lignans (1*-3*) with (2R,3R)-absolute configuration exhibited preferential cytotoxicity in a concentration-dependent manner with PC50 values of 0.54, 6.82, and 5.85 microM, respectively. Furthermore, the effect of (-)- and (+)-arctigenin was evaluated against the activation of Akt, which is a key process in the tolerance to nutrition starvation. Interestingly, only (-)-arctigenin (1*) strongly suppressed the activation of Akt.
CONCLUSIONS:
These results indicate that the (2R,3R)-absolute configuration of (-)-enantiomers should be required for the preferential cytotoxicity through the inhibition of Akt activation.
Yao Xue Xue Bao. 2001 Sep;36(9):669-71.
New biflavanones and bioactive compounds from Stellera chamaejasme L.[Pubmed:
12580104]
To study the chemical constituents of the root of Stellera chamaejasme L.
METHODS AND RESULTS:
Various column chromatographies on silica gel and RP-18 were employed for isolation and purification. Structures of compounds were elucidated by spectral analysis. Eight lignans and three biflavonoids possessing a C-3/C-3" linkage were isolated. They are ruixianglangdusu A (1) and B (2), 4',4'",5,5",7,7"-hexahydroxy-3,3"-biflavone (3), (+)-kusunokinin (4), lirioresinol-B (5), magnolenin C (6), (-)-pinoresinol monomethyl ether (7), (-)-pinoresinol (8), (+)-Matairesinol (9), isohinokinin (10) and (-)-eudesmin (11).
CONCLUSIONS:
Compounds 1 and 2 are new biflavanones, 1 is enantiomeric to known chamaejasmenin C, 4, 6, 8, 9, 10 and 11 were isolated from this plant for the first time, and 7 was isolated from natural resources for the first time. In vitro bioassays showed that 3 and 8 exhibited antibacterial activity, and 1, 2, 9 and 11 exhibited immunomodulatory activity.
Biosci Biotechnol Biochem. 2009 Aug;73(8):1718-21.
Inhibition of the discoloration of yellowtail dark muscle by lignan.[Pubmed:
19661688]
METHODS AND RESULTS:
The inhibitory effect of (-)-, (+)-Matairesinol and (-)-, (+)-secoisolariciresinol on the discoloration of dark muscle (chiai in Japanese) of two-year-old yellowtail (hamachi in Japanese) was evaluated by measuring the X and a(*) values.
CONCLUSIONS:
(-)-Matairesinol was most effective for retaining the red color of dark muscle in this experiment.