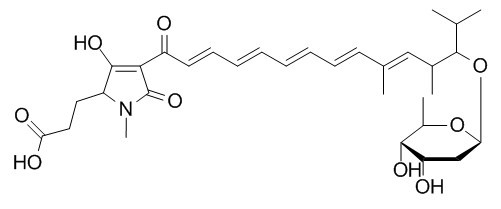
alpha-Lipomycin
Alpha-Lipomycin belongs to the classification of hybrid peptide-polyketide natural products. Alpha-Lipomycin is active against gram-positive organisms.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
South African Journal of Botany2024, 168:209-220.
J Lipid Res.2024, 65(10):100640.
J Nat Prod.2015, 78(6):1339-4
Molecules.2024, 29(21):5161.
Analytical methods2019, 11(6)
Research Square2021, 10.21203.
Phytochem Anal.2013, 24(5):493-503
Biotechnology and Bioprocess Engineering2024, 29:1048-1060.
Patanjali Research Foundation2024, ssrn.4807357
Planta Med.2019, 85(4):347-355
Related and Featured Products
Antimicrob Agents Chemother. 2006 Jun;50(6):2113-21.
Biosynthetic gene cluster for the polyenoyltetramic acid alpha-lipomycin.[Pubmed:
16723573]
The gram-positive bacterium Streptomyces aureofaciens Tü117 produces the acyclic polyene antibiotic alpha-Lipomycin.
METHODS AND RESULTS:
The entire biosynthetic gene cluster (lip gene cluster) was cloned and characterized. DNA sequence analysis of a 74-kb region revealed the presence of 28 complete open reading frames (ORFs), 22 of them belonging to the biosynthetic gene cluster. Central to the cluster is a polyketide synthase locus that encodes an eight-module system comprised of four multifunctional proteins. In addition, one ORF shows homology to those for nonribosomal peptide synthetases, indicating that alpha-Lipomycin belongs to the classification of hybrid peptide-polyketide natural products. Furthermore, the lip cluster includes genes responsible for the formation and attachment of d-digitoxose as well as ORFs that resemble those for putative regulatory and export functions. We generated biosynthetic mutants by insertional gene inactivation.
CONCLUSIONS:
By analysis of culture extracts of these mutants, we could prove that, indeed, the genes involved in the biosynthesis of lipomycin had been cloned, and additionally we gained insight into an unusual biosynthesis pathway.
Org Lett. 2015 Feb 6;17(3):628-31.
Discovery of a new family of Dieckmann cyclases essential to tetramic acid and pyridone-based natural products biosynthesis.[Pubmed:
25621700]
METHODS AND RESULTS:
Bioinformatic analyses indicate that TrdC, SlgL, LipX2, KirHI, and FacHI belong to a group of highly homologous proteins involved in biosynthesis of actinomycete-derived tirandamycin B, streptolydigin, α-lipomycin, kirromycin, and factumycin, respectively. However, assignment of their biosynthetic roles has remained elusive.
CONCLUSIONS:
Gene inactivation and complementation, in vitro biochemical assays with synthetic analogues, point mutations, and phylogenetic tree analyses reveal that these proteins represent a new family of Dieckmann cyclases that drive tetramic acid and pyridone scaffold biosynthesis.
Angew Chem Int Ed Engl. 2014 Jul 7;53(28):7328-34.
α- and β-Lipomycin: total syntheses by sequential stille couplings and assignment of the absolute configuration of all stereogenic centers.[Pubmed:
24895187]
40 years ago spectroscopy, derivatization, and degradation revealed the structures of alpha-Lipomycin and its aglycon β-lipomycin except for the configurations of their side-chain stereocenters.
METHODS AND RESULTS:
We synthesized all relevant β-lipomycin candidates: the (12R,13S) isomer has the same specific rotational value as the natural product. By the same criterion the (12R,13S)-configured D-digitoxide is identical to alpha-Lipomycin.
CONCLUSIONS:
We double-checked our assignments by degrading alpha-Lipomycin and β-lipomycin to the diesters 33 and 34 and proving their 3D structures synthetically.