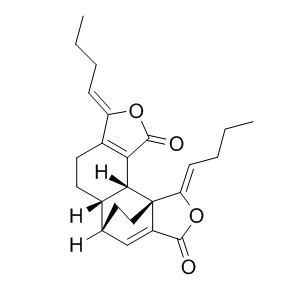
Levistilide A
Traditional formula compatibility of Danggui-Shaoyao-San could significantly enhance levistilide A bioavailability compared with levistilide A alone and Rhizoma Chuanxiong.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2022, 23(24):16000.
Front Pharmacol.2020, 11:566490.
Korean j.of Pharm.2017, 70-76
LWT2020, 130:109535
Nat Commun.2025, 16(1):4121.
Pharmacol Res.2020, 161:105205.
Antioxidants (Basel).2023, 12(5):1111.
Int J Mol Sci.2024, 25(15):8101.
Appl. Sci.2022, 12(4), 2032.
Int J Mol Sci.2020, 21(8):2790.
Related and Featured Products
Cell Physiol Biochem . 2017;42(3):929-938.
Levistolide A Induces Apoptosis via ROS-Mediated ER Stress Pathway in Colon Cancer Cells[Pubmed:
28662507]
Abstract
Background/aims: Colorectal cancer (CRC) is one of the leading causes of cancer-related death worldwide. Levistolide A (LA), a natural compound isolated from the traditional Chinese herb Ligusticum chuanxiong Hort, is used for treating cancer. In this study, we investigated the anticancer effect of LA in HCT116 and its isogenic p53-/- colon cancer cells, as well as the underlying mechanisms.
Methods: MTT assay was used to evaluate the effect of LA on the viability of cancer cells. Apoptosis and reactive oxygen species (ROS) production by the cells were determined by flow cytometry. Protein expression was detected by western blotting.
Results: The results showed that LA inhibited viability and caused apoptosis of both wild-type and p53-/- HCT116 cells. LA was able to trigger production of ROS and endoplasmic reticulum (ER) stress. Inhibition of ROS using N-acetylcysteine abrogated LA-induced ER stress and apoptosis, as well as the reduction in cancer cell viability.
Conclusion: Our results indicate that LA causes apoptosis of colon cancer cells via ROS-mediated ER stress pathway. It will be interesting to develop the natural compound for chemotherapy of cancers such as CRC.
Keywords: Apoptosis; ER stress; Levistolide A; ROS.
J Chromatogr Sci. 2015 Feb 5. pii: bmu224.
Study on Pharmacokinetics of Three Preparations from Levistolide A by LC-MS-MS.[Pubmed:
25657289]
A rapid sensitive analytical method was established and validated to investigate levistolide A in rat plasma by liquid chromatography-tandem mass spectrometry operated in the positive ion mode.
METHODS AND RESULTS:
Levistilide A (LA) and internal standard (IS) andrographolide (AD), mixed with the plasma sample, were separated on a reversed phase Spursil™ C18 5 μm column. The precursor/product transitions (m/z) were 398.5/381.3 for Levistilide A and (m/z) 368.0/351.1 for AD. The calibration curve was linear over the range from 5 to 1,250 ng/mL for oral administration and 10-4,000 for intravenous administration with a correlation coefficient (r) ≥0.9993. The lower limit of quantification was 5 ng/mL for Levistilide A in plasma. The inter- and intra-day accuracy and precision were less than ±15% of the relative standard deviation. In this study, the developed method is successfully applied to the comparative pharmacokinetic study of Levistilide A in rats after oral administration of Levistilide A alone, Rhizoma Chuanxiong, and Danggui-Shaoyao-San along with the bioavailability study of Levistilide A in rats.
CONCLUSIONS:
Our study shows that low bioavailability (7.5%) is observed after oral administration of Levistilide A .Traditional formula compatibility of Danggui-Shaoyao-San could significantly enhance Levistilide A bioavailability compared with Levistilide A alone and Rhizoma Chuanxiong.