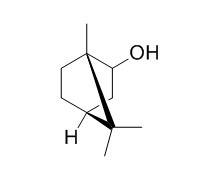
Isoborneol
Isoborneol shows neuroprotective, antioxidant, and repellent effects, it shows dual viricidal activity against herpes simplex virus 1 (HSV-1).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Toxicol In Vitro.2024, 99:105876.
Eur J Pharmacol.2021, 899:174010.
Biomedicines.2021, 9(8):954.
Process Biochemistry2019, 87:213-220
J of Food Quality2020, 8851285.
PLoS One.2020, 15(2):e0220084.
J Nat Med.2017, 71(4):745-756
Journal of Apiculture2019, 34(2):131-136
The University of Manitoba2021, 35690.
BMC Complement Altern Med.2018, 18(1):303
Related and Featured Products
Antiviral Res. 1999 Sep;43(2):79-92.
Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1.[Pubmed:
10517310]
Isoborneol, a monoterpene and a component of several plant essential oils, showed dual viricidal activity against herpes simplex virus 1 (HSV-1).
METHODS AND RESULTS:
First, it inactivated HSV-1 by almost 4 log10 values within 30 min of exposure, and second, Isoborneol at a concentration of 0.06% completely inhibited viral replication, without affecting viral adsorption. Isoborneol did not exhibit significant cytotoxicity at concentrations ranging between 0.016% and 0.08% when tested against human and monkey cell lines. Isoborneol specifically inhibited glycosylation of viral polypeptides based on the following data: (1) the mature fully glycosylated forms of two viral glycoproteins gB and gD were not detected when the virus was replicated in the presence of Isoborneol, (2) no major changes were observed in the glycosylation pattern of cellular polypeptides between untreated and Isoborneol treated Vero cells, (3) Isoborneol did not affect the glycosylation of gB produced from a copy of the gB gene resident in the cellular genome, and (4) other monoterpenes such as 1,8-cineole and borneol, a stereoisomer of Isoborneol, did not inhibit HSV-1 glycosylation.
J Econ Entomol. 2003 Aug;96(4):1267-74.
Repellent effects of isoborneol on subterranean termites (Isoptera: Rhinotermitidae) in soils of different composition[Pubmed:
14503600]
METHODS AND RESULTS:
The repellence of the plant-derived bicyclic monoterpenoid Isoborneol on subterranean termites was assessed in short-term laboratory bioassays. Depending on concentration, application of Isoborneol to different soil types was efficient in creating repellent soil barriers, which were not penetrated by workers of Reticulitermes santonensis De Feytaud or R. flavipes Kollar within 2 wk after adding the substance to the substrate. Isoborneol-treated barriers did not affect termite survival. The bioavailability of the active ingredient decreased with increasing clay content of the soil.
CONCLUSIONS:
Evaporation of Isoborneol from treated soil increased with increasing particle size of the substrate and could be reduced by covering the soil surface.
Cell Physiol Biochem. 2007;20(6):1019-32.
Protective effect of (+/-) isoborneol against 6-OHDA-induced apoptosis in SH-SY5Y cells.[Pubmed:
17975304 ]
Oxidative stress caused by dopamine (DA) may play an important role in the pathogenesis of Parkinson's disease (PD). (+/-) Isoborneol is a monoterpenoid alcohol present in the essential oils of numerous medicinal plants and is a known antioxidant.
METHODS AND RESULTS:
In this study, we investigated the neuroprotective effect of Isoborneol against 6-hydroxydopamine (6-OHDA)-induced cell death in human neuroblastoma SH-SY5Y cells. Pretreatment of SH-SY5Y cells with Isoborneol significantly reduced 6-OHDA-induced generation of reactive oxygen species (ROS) and 6-OHDA-induced increases in intracellular calcium. Furthermore, apoptosis induced by 6-OHDA was reversed by Isoborneol treatment. Isoborneol protected against 6-OHDA-induced increases in caspase-3 activity and cytochrome C translocation into the cytosol from mitochondria. Isoborneol prevented 6-OHDA from decreasing the Bax/Bcl-2 ratio. We also observed that Isoborneol decreased the activation of c-Jun N-terminal kinase and induced activation of protein kinase C (PKC) which had been suppressed by 6-OHDA.
CONCLUSIONS:
Our results indicate that the protective function of Isoborneol is dependent upon its antioxidant potential and strongly suggest that Isoborneol may be an effective treatment for neurodegenerative diseases associated with oxidative stress.
Asian Journal of Chemistry, 2014, 26(4):997-1001.
Determination of (-)-Borneol, Camphor and Isoborneol in Blumea balsamifera (L.) DC. Leaves by Simultaneous Ultrasonic and Microwave Assisted Extraction and Gas Chromatography[Reference:
WebLink]
METHODS AND RESULTS:
In present work, simultaneous ultrasonic and microwave assisted extraction followed by GC-FID was developed for quantitative analysis of the bioactive components of (-)-borneol, camphor and Isoborneol in Blumea balsamifera leaves. After systematical investigation, the optimal experimental parameters microwave power (100 W) and extraction time (30 s) were investigated. The optimized method provided satisfactory linearity, precision, stability and recovery. The proposed method was applied for determination of three target compounds in leaves samples from 14 different times of harvest period. The variations of three target compounds were monitored and the result demonstrates (-)-borneol content of leaves was higher level from mid-October to next January.
CONCLUSIONS:
It has been shown that the proposed ultrasonic and microwave assisted extraction-GC-FID is a simple, rapid and reliable method for quantitative analysis of (-)-borneol, camphor and Isoborneol in B. balsamifera leaves and a potential tool for quality assessment of B. balsamifera leaves.