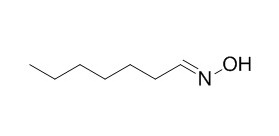
Heptanal oxime
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2019, 24(6):E1155
Pharmaceutics.2022, 14(5):945.
Research Square2022, rs.3.rs-1948239
Cell Metab.2020, S1550-4131(20)30002-4
J Pain Res.2022, 15:3469-3478.
Molecules.2020, 25(3):734
J of Liquid Chromatography & Related Technologies2024, 47(1-5):14-25.
TCI CO.2019, US20190151257A1
Nutrients.2023, 15(13):2960.
Comput Biol Chem.2019, 83:107096
Related and Featured Products
The Journal of Physical Chemistry B, 1972, 76(13):1819-1824.
Electron spin resonance study of x-irradiated heptanal oxime trapped in a urea inclusion crystal. Evidence of a bimolecular reaction product.[Reference:
WebLink]
METHODS AND RESULTS:
Free radicals trapped in X-irradiated Heptanal oxime-urea inclusion crystals were investigated using electron spin resonance techniques. In addition to an. iminoxy radical, R-CH=NO·, two secondary radicals were observed. Spectral simulations and a table of coupling constants and g values are presented. Esr spectra and selective deuteration suggest that the secondary radicals are of the type R-N-R′ and RR′NO .