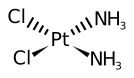
Cisplatin
Cisplatin is a antineoplastic chemotherapy drug which works by cross-linking with DNA and causing DNA damage in cancer cells, it can enhance the cell-killing effect of radiation, an effect whose intensity varies with the schedule of administration.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Industrial Crops and Products2022, 188:115596.
Curr Res Virol Sci.2022, 3:100019.
J Korean Society of Food Science & Nutrition2021, 50(9): 962-970
J Agric Food Chem.2024, 72(42):23183-23195
J Ethnopharmacol.2018, 210:88-94
RSC Advances2017, 86
Kor.J.Herbol.2024, 39(3):23-35
Molecules.2023, 28(13):4907.
Pest Manag Sci.2023, 79(8):2675-2685.
Int J Mol Sci.2024, 25(1):616.
Related and Featured Products
N Engl J Med. 1992 Feb 20;326(8):524-30.
Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer.[Pubmed:
1310160 ]
Cisplatin (cis-diamminedichloroplatinum) has been reported to enhance the cell-killing effect of radiation, an effect whose intensity varies with the schedule of administration.
METHODS AND RESULTS:
We randomly assigned 331 patients with nonmetastatic inoperable non-small-cell lung cancer to one of three treatments: radiotherapy for two weeks (3 Gy given 10 times, in five fractions a week), followed by a three-week rest period and then radiotherapy for two more weeks (2.5 Gy given 10 times, five fractions a week); radiotherapy on the same schedule, combined with 30 mg of Cisplatin per square meter of body-surface area, given on the first day of each treatment week; or radiotherapy on the same schedule, combined with 6 mg of Cisplatin per square meter, given daily before radiotherapy.
Survival was significantly improved in the radiotherapy-daily-Cisplatin group as compared with the radiotherapy group (P = 0.009): survival in the radiotherapy-daily-Cisplatin group was 54 percent at one year, 26 percent at two years, and 16 percent at three years, as compared with 46 percent, 13 percent, and 2 percent, respectively, in the radiotherapy group. Survival in the radiotherapy-weekly-Cisplatin group was intermediate (44 percent, 19 percent, and 13 percent) and not significantly different from survival in either of the other two groups. The survival benefit of daily combined treatment was due to improved control of local disease (P = 0.003). Survival without local recurrence was 59 percent at one year and 31 percent at two years in the radiotherapy-daily-Cisplatin group; 42 percent and 30 percent, respectively, in the radiotherapy-weekly-Cisplatin group; and 41 percent and 19 percent, respectively, in the radiotherapy group. Cisplatin induced nausea and vomiting in 86 percent of the patients given it weekly and in 78 percent of those given it daily; these effects were severe in 26 percent and 28 percent, respectively.
CONCLUSIONS:
Cisplatin, given daily in combination with the radiotherapy described here to patients with nonmetastatic but inoperable non-small-cell lung cancer, improved rates of survival and control of local disease at the price of substantial side effects.
Food Chem Toxicol. 2012 May;50(5):1675-9.
Regulation of renal organic anion and cation transporters by thymoquinone in cisplatin induced kidney injury.[Pubmed:
22414646]
In previous studies, we have demonstrated the biological activity of thymoquinone (TQ), an active compound extracted from the Nigella sativa plant, against Cisplatin-induced neurotoxicity. Recenty, it was observed that there is an inherent lack in regulation of renal organic anion and cation transporters in Cisplatin-induced nephrotoxicity.
METHODS AND RESULTS:
Here, we report, for the first time, the effect of TQ on alterations in the renal expression of organic anion transporters (OATs) and organic cation transporters (OCTs), as well as multidrug resistance-associated proteins (MRPs) in rats treated with Cisplatin. Twenty-eight 8-week-old male Wistar rats were divided into four groups of control, TQ treated (10 mg/kg b.w. in drinking water for 5 days), Cisplatin (7 mg/kg b.w., i.p.) and TQ and Cisplatin combination treatment. Cisplatin-induced malondialdehyde (MDA) and 8-isoprostane increase was found to be markedly reduced in rats treated with TQ. In Cisplatin only treated rats, the induced renal injury increased protein levels of the efflux transporters MRP2 and MRP4 while expression of OAT1, OAT3, OCT1 and OCT2 was reduced. In combination TQ- and Cisplatin-treated rats, expression of MRP2 and MRP4 proteins was decreased in the kidneys. Conversely, TQ treatment increased levels of OCT1, OCT2, OAT1 and OAT3 and decreased levels of 8-isoprostane and MDA levels in Cisplatin-treated rats.
CONCLUSIONS:
In conclusion, the present study shows that the TQ synergizes with its nephroprotective effect against Cisplatin-induced acute kidney injury in rats.
Jpn J Cancer Res. 1993 Feb;84(2):203-7.
Enhanced antitumor efficacy of a combination of CPT-11, a new derivative of camptothecin, and cisplatin against human lung tumor xenografts.[Pubmed:
8385085]
The objective of this study was to evaluate the antitumor efficacy of combined use of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin (CPT-11) and Cisplatin (CDDP).
METHODS AND RESULTS:
The antitumor activities of CPT-11, CDDP and their combination against 3 human lung tumor xenografts were estimated using congenitally athymic BALB/c (nu/nu) mice. The doses were 47 mg/kg for CPT-11 and 6 mg/kg for CDDP on days 1, 5 and 9. In combination therapy, half of the single dosage of each agent was used. The doses were administered intraperitoneally. The antitumor activity and toxicity were evaluated in terms of the tumor volume and body weight change of mice, respectively. The combination therapy resulted in a statistically significant tumor regression compared to the use of only CPT-11 or CDDP in two tumor xenografts out of three. The toxicity of the combination therapy was no higher than that of CPT-11 or CDDP alone.
CONCLUSIONS:
These results suggest that the antitumor activity of the combination of CPT-11 and CDDP is superior to that of CPT-11 or CDDP alone.