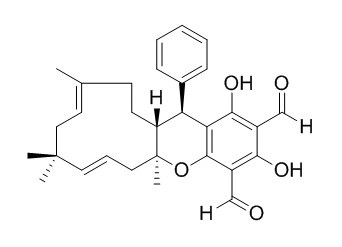
Guajadial B
Guajadial B acts as a Top1 catalytic inhibitor and delays Top1 poison-mediated DNA damage. Guajadial B shows cytotoxicities against five human cancer cell lines, it is the most effective having an IC50 value of 150 nM toward A549 cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytother Res.2019, 33(3):676-689
Chulalongkorn University2024, ssrn.4716057.
Heliyon.2023, e12684.
Phytother Res.2022, 10.1002:ptr.7602.
Antioxidants (Basel).2019, 8(8):E307
J Mol Recognit.2020, 33(2):e2819
Biomedicines.2022, 10(2):463.
Separation Science Plus2022, sscp.202200048.
Int J Mol Sci.2020, 21(9):3239.
Front Pharmacol.2020, 11:251.
Related and Featured Products
J Agric Food Chem. 2017 Jun 21;65(24):4993-4999.
Meroterpenoids with Antitumor Activities from Guava (Psidium guajava).[Pubmed:
28578580]
Psidium guajava L., a species native to South America, has been widely cultivated in the tropical and subtropical areas of China for its popular fruits.
METHODS AND RESULTS:
The preliminary analysis by liquid chromatography-ultraviolet (LC-UV) indicated the presence of meroterpenoids in the fruits of P. guajava (guava). Subsequent fractionation of the petroleum ether extract resulted in the identification of two new meroterpenoids, psiguajavadials A (1) and B (2), together with 14 previously described meroterpenoids (3-16). Their structures were fully elucidated by comprehensive spectroscopic techniques and theoretical calculations. All of the meroterpenoids showed cytotoxicities against five human cancer cell lines, with Guajadial B (12) being the most effective having an IC50 value of 150 nM toward A549 cells. Furthermore, biochemical topoisomerase I (Top1) assay revealed that psiguajavadial A (1), psiguajavadial B (2), Guajadial B (12), guajadial C (14), and guajadial F (16) acted as Top1 catalytic inhibitors and delayed Top1 poison-mediated DNA damage.
CONCLUSIONS:
The flow cytometric analysis indicated that the new meroterpenoids psiguajavadials A (1) and B (2) could induce apoptosis of HCT116 cells. These data suggest that meroterpenoids from guava fruit could be used for the development of antitumor agents.
Org Lett. 2012 Dec 7;14(23):5936-9.
Isolation and biomimetic synthesis of (±)-guajadial B, a novel meroterpenoid from Psidium guajava.[Pubmed:
23163238]
METHODS AND RESULTS:
(±)-Guajadial B (1), an unusual humulene-based meroterpenoid, was isolated as a racemate from the leaves of Psidium guajava, collected from Vietnam. The structure of this novel secondary metabolite was established on the basis of extensive analysis of NMR spectra and confirmed by biomimetic synthesis in a domino three-component coupling reaction.