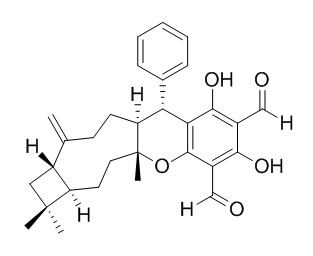
Guajadial
Guajadial shows α-glucosidase inhibitory activities significantly and its activity was better than that of Acarbose.Guajadial may act as Selective Estrogen Receptors Modulators (SERMs), therefore holding significant potential for anticancer therapy, it exhibits potent inhibitory effects on the growth of human hepatoma cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmacol Rep.2020, 72(2):472-480.
Molecules2022, 27(9):2613.
Sci Rep.2021, 11(1):10931.
Acta Pharm Sin B.2024, 14(4):1772-1786.
Int J Mol Sci.2021, 22(10):5181.
J Sci Food Agric.2022, 102(4):1628-1639
Applied Biological Chemistry2020, 63:37.
Sci Rep.2019, 9(1):4646
Molecules.2019, 24(7):E1290
Sci Rep.2020, 10:4495(2020)
Related and Featured Products
Curr Med Chem. 2014;21(20):2322-30.
In vitro, in vivo and in silico analysis of the anticancer and estrogen-like activity of guava leaf extracts.[Pubmed:
24438525]
Anticancer drug research based on natural compounds enabled the discovery of many drugs currently used in cancer therapy.
METHODS AND RESULTS:
Here, we report the in vitro, in vivo and in silico anticancer and estrogen-like activity of Psidium guajava L. (guava) extracts and enriched mixture containing the meroterpenes Guajadial, psidial A and psiguadial A and B. All samples were evaluated in vitro for anticancer activity against nine human cancer lines: K562 (leukemia), MCF7 (breast), NCI/ADR-RES (resistant ovarian cancer), NCI-H460 (lung), UACC-62 (melanoma), PC-3 (prostate), HT-29 (colon), OVCAR-3 (ovarian) and 786-0 (kidney). Psidium guajava's active compounds displayed similar physicochemical properties to estradiol and tamoxifen, as in silico molecular docking studies demonstrated that they fit into the estrogen receptors (ERs). The meroterpene-enriched fraction was also evaluated in vivo in a Solid Ehrlich murine breast adenocarcinoma model, and showed to be highly effective in inhibiting tumor growth, also demonstrating uterus increase in comparison to negative controls.
CONCLUSIONS:
The ability of Guajadial, psidial A and psiguadials A and B to reduce tumor growth and stimulate uterus proliferation, as well as their in silico docking similarity to tamoxifen, suggest that these compounds may act as Selective Estrogen Receptors Modulators (SERMs), therefore holding significant potential for anticancer therapy.
Journal of Hunan University of Chinese Medicine, 2014 , 34 (8) :17-20.CFN97539
Sesquiterpenoids with α-Glucosidase Inhibitory Activities from the Leaves of Psidium guajava Linn[Reference:
WebLink]
To study the α-glucosidase inhibitory active compounds from the ethyl acetate extract of guava leaves.
METHODS AND RESULTS:
The compounds were separated from the ethyl acetate extract by repeating silica gel and Sephadex LH-20 column chromatography. The 1H-NMR,13C-NMR techniques were used to identify the chemical structures. The α-glucosidase inhibitory activities were tested by colorimetry. Three special meroterpenoids were isolated and identified as psidials A, psiguadial A and Guajadial. Guajadial showed α-glucosidase inhibitory activities significantly and its activity was better than that of Acarbose.
CONCLUSIONS:
Guajadial as one of the α-glucosidase inhibitory active compound from Guava leaves was reported for the first time.
Org Lett. 2010 Apr 16;12(8):1676-9.
A short biomimetic synthesis of the meroterpenoids guajadial and psidial A.[Pubmed:
20235528]
The biosynthesis of the meroterpenoid Guajadial was previously hypothesized to occur via a hetero-Diels-Alder reaction between caryophyllene and an o-quinone methide.
METHODS AND RESULTS:
This hypothesis has been verified via the biomimetic synthesis of Guajadial and psidial A in an aqueous three-component coupling reaction, between caryophyllene, benzaldehyde, and diformylphloroglucinol.
Org Lett. 2007 Nov 22;9(24):5135-8.
Guajadial: an unusual meroterpenoid from guava leaves Psidium guajava.[Pubmed:
17985919]
Guajadial (1), a novel caryophyllene-based meroterpenoid, was isolated from the Leaves of Psidium guajava (guava).
METHODS AND RESULTS:
The structure and relative stereochemistry of Guajadial (1) were elucidated by extensive spectroscopic analysis. A possible biosynthetic pathway for Guajadial was proposed.
Org Lett. 2010 Nov 5;12(21):5040-3.
Psiguadials A and B, two novel meroterpenoids with unusual skeletons from the leaves of Psidium guajava.[Pubmed:
20929258 ]
METHODS AND RESULTS:
Psiguadials A (1) and B (2), two novel sesquiterpenoid-diphenylmethane meroterpenoids with unusual skeletons, along with a pair of known epimers, psidial A (3) and Guajadial (4), were isolated from the leaves of Psidium guajava. Their structures with absolute configurations were elucidated by means of NMR, X-ray diffraction, and quantum chemical CD calculation.
CONCLUSIONS:
Compounds 1, 2, and 4 exhibited potent inhibitory effects on the growth of human hepatoma cells.