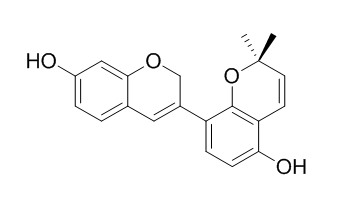
Glabrene
Glabrene, Liquiritin apioside, neolicuroside, and 18β-glycyrrhetic acid are the predominant phenolic derivatives partitioning at the interface and most likely the major contributors to the notable synergistic antioxidant activity when coupled with pea protein hydrolysates (PPHs).Glabrene has estrogen-like activity, it can stimulate DNA synthesis in human endothelial cells (ECV-304; E304) and has a bi-phasic effect on proliferation of human vascular smooth muscle cells (VSMC). Glabrene and isoliquiritigenin may serve as candidates for skin-lightening agents, they exert varying degrees of inhibition on tyrosinase-dependent melanin biosynthesis.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Hum Exp Toxicol.2023, 42:9603271221145386.
Industrial Crops and Products2022, 188:115596.
Korean J. Food Sci. & Technol.2022, 54(2):241-246
Food Res Int.2017, 96:40-45
Foods.2023, 12(19):3647.
Faculty of Chem. & Nat. Resource Eng.2014, 62
International. J. of Food Properties 2017, 20:S131-S140
Molecules.2019, 24(7):E1290
Pharmacol Rep.2019, 71(2):289-298
J Ethnopharmacol.2020, 254:112733.
Related and Featured Products
J Agric Food Chem. 2003 Feb 26;51(5):1201-7.
Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots.[Pubmed:
12590456 ]
Tyrosinase is known to be a key enzyme in melanin biosynthesis, involved in determining the color of mammalian skin and hair. Various dermatological disorders, such as melasama, age spots, and sites of actinic damage, arise from the accumulation of an excessive level of epidermal pigmentation. The inadequacy of current therapies to treat these conditions as well as high cytotoxicity and mutagenicity, poor skin penetration, and low stability of formulations led us to seek new whitening agents to meet the medical requirements for depigmenting agents.
METHODS AND RESULTS:
The inhibitory effect of licorice extract on tyrosinase activity was higher than that expected from the level of glabridin in the extract. This led us to test for other components that may contribute to this strong inhibitory activity. Results indicated that Glabrene and isoliquiritigenin (2',4',4-trihydroxychalcone) in the licorice extract can inhibit both mono- and diphenolase tyrosinase activities. The IC(50) values for Glabrene and isoliquiritigenin were 3.5 and 8.1 microM, respectively, when tyrosine was used as substrate. The effects of Glabrene and isoliquiritigenin on tyrosinase activity were dose-dependent and correlated to their ability to inhibit melanin formation in melanocytes.
CONCLUSIONS:
This is the first study indicating that Glabrene and isoliquiritigenin exert varying degrees of inhibition on tyrosinase-dependent melanin biosynthesis, suggesting that isoflavenes and chalcones may serve as candidates for skin-lightening agents.
J Steroid Biochem Mol Biol. 2004 Jul;91(3):147-55.
Estrogen-like activity of licorice root constituents: glabridin and glabrene, in vascular tissues in vitro and in vivo.[Pubmed:
15276622 ]
Post-menopausal women have higher incidence of heart diseases compared to pre-menopausal women, suggesting a protective role for estrogen. The recently Women's Health Initiative (WHI) randomized controlled trial concluded that the overall heart risk exceeded benefits from use of combined estrogen and progestin as hormone replacement therapy for an average of five years among healthy postmenopausal US women. Therefore, there is an urgent need for new agents with tissue-selective activity with no deleterious effects.
METHODS AND RESULTS:
In the present study, we tested the effects on vascular tissues in vitro and in vivo of two natural compounds derived from licorice root: glabridin, the major isoflavan, and Glabrene, an isoflavene, both demonstrated estrogen-like activities. Similar to estradiol-17beta (E2), glabridin (gla) stimulated DNA synthesis in human endothelial cells (ECV-304; E304) and had a bi-phasic effect on proliferation of human vascular smooth muscle cells (VSMC). Raloxifene inhibited gla as well as E2 activities. In animal studies, both intact females or after ovariectomy, gla similar to E2 stimulated the specific activity of creatine kinase (CK) in aorta (Ao) and in left ventricle of the heart (Lv). Glabrene (glb), on the other hand, had only the stimulatory effect on DNA synthesis in vascular cells, with no inhibition by raloxifene, suggesting a different mechanism of action. To further elucidate the mechanism of action of glb, cells were pre-incubated with glb and then exposed to either E2 or to gla; the DNA stimulation at low doses was unchanged but there was abolishment of the inhibition of VSMC cell proliferation at high doses as well as inhibition of CK stimulation by both E2 and by gla. We conclude that glb behaved differently than E2 or gla, but similarly to raloxifene, being a partial agonist/antagonist of E2. Glabridin, on the other hand, demonstrated only estrogenic activity.
CONCLUSIONS:
Therefore, we suggest the use of glb with or without E2 as a new agent for modulation of vascular injury and atherogenesis for the prevention of cardiovascular diseases in post-menopausal women.
J Agric Food Chem. 2014 Aug 13;62(32):8204-13.
Synergy of licorice extract and pea protein hydrolysate for oxidative stability of soybean oil-in-water emulsions.[Pubmed:
25058384 ]
Previously developed radical-scavenging pea protein hydrolysates (PPHs) prepared with Flavourzyme (Fla-PPH) and Protamex (Pro-PPH) were used as cosurfactants with Tween 20 to produce soybean oil-in-water (O/W) emulsions, and the suppression of lipid oxidation was investigated.
METHODS AND RESULTS:
Both PPHs significantly retarded oxidation (P < 0.05) of the emulsions when stored at 37 °C for 14 days. Electron microscopy revealed an interfacial peptidyl membrane around oil droplets, which afforded steric restrictions to oxidation initiators. When licorice extract (LE) was also used in emulsion preparation, a remarkable synergistic oxidation inhibition was observed with both PPHs. LE adsorbed onto oil droplets either directly or through associating with PPH to produce a thick and compact interfacial membrane enabling the defense against oxygen species.
CONCLUSIONS:
Liquiritin apioside, neolicuroside, Glabrene, and 18β-glycyrrhetic acid were the predominant phenolic derivatives partitioning at the interface and most likely the major contributors to the notable synergistic antioxidant activity when coupled with PPHs.