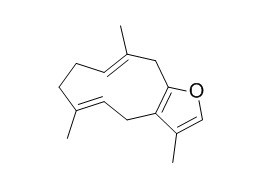
Furanodiene
Furanodiene has anti-inflammatory and antioxidant activities, it is active against gram-positive bacteria and Candida albicans. Furanodiene suppresses breast cancer cell growth both in vitro and in vivo and could be a new lead compound for breast cancer chemotherapy, it presents synergistic anti-proliferative activity with paclitaxel via altering cell cycle and integrin signaling in 95-D lung cancer cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
RSC Advances2017, 86
Int J Pharmacol2020, 16:1-9
The Japan Society for Analytical Chemistry2018, 67(4):201-206
Naunyn Schmiedebergs Arch Pharmacol.2021, 394(1):107-115.
Curr Med Sci.2024, 44(2):355-368.
Molecules.2019, 24(11):E2102
Int J Mol Sci.2017, 19(1)
Journal of Mushroom2024, 22(4):192-198
Plants (Basel).2020, 9(11):1535.
Neurochem Int.2018, 121:114-124
Related and Featured Products
Cell Physiol Biochem. 2012;30(3):778-90.
Furanodiene, a natural product, inhibits breast cancer growth both in vitro and in vivo.[Pubmed:
22854281]
Previous studies have reported that the Curcuma wenyujin Y.H. Chen et C. Ling extract, which has a high Furanodiene content, showed anti-cancer effects in breast cancer cells in vitro. The present study was designed to evaluate the in vitro and in vivo anti-cancer activity of Furanodiene.
METHODS AND RESULTS:
The in vitro effects of Furanodiene were examined on two human breast cancer cell lines, MCF-7 and MDA-MB-231 cells. Assays of proliferation, LDH release, mitochondrial membrane potential (ΔΨm), cell cycle distribution, apoptosis and relevant signaling pathways were performed. The in vivo effect was determined with MCF7 tumor xenograft model in nude mice. Furanodiene significantly inhibited the proliferation and increased the LDH release in both cell lines in a dose-dependent manner. ΔΨm depolarization, chromatin condensation, and DNA fragmentation were also observed after Furanodiene treatment. Furanodiene dose-dependently induced cell cycle arrest at the G0/G1 phase. The protein expressions of p-cyclin D1, total cyclin D1, p-CDK2, total CDK2, p-Rb, total Rb, Bcl-xL, and Akt were significantly inhibited by Furanodiene, whereas the protein expressions of Bad and Bax, and the proteolytic cleavage of caspase-9, caspase-7, and poly-ADP-ribose polymerase (PARP) were dramatically increased. Furthermore, the z-VAD-fmk markedly reversed the Furanodiene-induced cell cytotoxicity, the proteolytic cleavage of caspase-9, and DNA fragmentation but did not affect the proteolytic cleavage of PARP, whereas the Akt inhibitor VIII increased the Furanodiene-induced cytotoxicity and PARP cleavage. In addition, Furanodiene dose-dependently suppressed the tumor growth in vivo, achieving 32% and 54% inhibition rates after intraperitoneal injection of 15 mg/kg and 30 mg/kg, respectively.
CONCLUSIONS:
Taken together, we concluded that Furanodiene suppresses breast cancer cell growth both in vitro and in vivo and could be a new lead compound for breast cancer chemotherapy.
Mem Inst Oswaldo Cruz. 2015 Feb;110(1):106-13.
Therapeutic switching: from antidermatophytic essential oils to new leishmanicidal products.[Pubmed:
25742270 ]
This study examined whether the antidermatophytic activity of essential oils (EOs) can be used as an indicator for the discovery of active natural products against Leishmania amazonensis.
The aerial parts of seven plants were hydrodistilled.
METHODS AND RESULTS:
Using broth microdilution techniques, the obtained EOs were tested against three strains of dermatophytes (Trichophyton mentagrophytes, Microsporum gypseum and Microsporum canis). To compare the EOs antifungal and antiparasitic effects, the EOs activities against axenic amastigotes of L. amazonensis were concurrently evaluated. For the most promising EOs, their antileishmanial activities against parasites infecting peritoneal macrophages of BALB/c mice were measured. The most interesting antifungal candidates were the EOs from Cymbopogon citratus, Otacanthus azureus and Protium heptaphyllum, whereas O. azureus, Piper hispidum and P. heptaphyllum EOs exhibited the lowest 50% inhibitory concentration (IC50) values against axenic amastigotes, thus revealing a certain correspondence between both activities.
The P. hispidum EO was identified as the most promising product in the results from the infected macrophages model (IC50: 4.7 μg/mL, safety index: 8).
CONCLUSIONS:
The most abundant compounds found in this EO were sesquiterpenes, notably curzerene and Furanodiene. Eventually, the evaluation of the antidermatophytic activity of EOs appears to be an efficient method for identifying new potential drugs for the treatment of L. amazonensis.
Phytother Res. 2014 Feb;28(2):296-9.
Furanodiene presents synergistic anti-proliferative activity with paclitaxel via altering cell cycle and integrin signaling in 95-D lung cancer cells.[Pubmed:
23554049]
Furanodiene (FUR) is a natural terpenoid isolated from Rhizoma Curcumae, a well-known Chinese medicinal herb that presents anti-proliferative activities in several cancer cell lines. Recently, we found that the combined treatment of FUR with paclitaxel (TAX) showed synergetic anti-proliferative activities in 95-D lung cancer cells.
METHODS AND RESULTS:
Herein, we showed that FUR reduced the cell numbers distributed in mitosis phase induced by TAX while increased those in G1 phase. The protein levels of cyclin D1, cyclin B1, CDK6 and c-Myc were all down-regulated in the group of combined treatment. The dramatically down-regulated expression of integrin β4, focal adhesion kinase and paxillin might partially contribute to the synergic effect.
CONCLUSIONS:
Though FUR alone obviously induced endoplasmic reticulum stress, this signaling pathway may not contribute to the synergetic anti-proliferative effect as the protein expression of CHOP and BIP was similar in FUR alone and combined treatment group.
Nat Prod Res. 2006 Jun;20(7):680-5.
Anti-inflammatory sesquiterpenes from Curcuma zedoaria.[Pubmed:
16901812]
METHODS AND RESULTS:
From the methanolic extract of the rhizome of Curcuma zedoaria, we isolated anti-inflammatory sesquiterpene Furanodiene (1) and furanodienone (2) along with new sesquiterpene compound 3 and known eight sesquiterpenes, zederone (4), curzerenone (5), curzeone (6), germacrone (7), 13-hydroxygermacrone (8), dehydrocurdione (9), curcumenone (10), and zedoaronediol (11). Their structures were elucidated on the basis of spectroscopic data. The anti-inflammatory effect of isolated components on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation of mouse ears were examined.
CONCLUSIONS:
Compounds 1 and 2 suppressed the TPA-induced inflammation of mouse ears by 75% and 53%, respectively, at a dose of 1.0 micromol. Their activities are comparable to that of indomethacin, the normally used anti-inflammatory agent.
J Med Assoc Thai. 2014 Aug;97 Suppl 8:S64-9.
Cholinesterase inhibitory activities of Apai-sa-le recipe and its ingredients.[Pubmed:
25518295]
Acetylcholinesterase and butyrylcholoinesterase inhibitors are well-known drugs commonly used in the treatment ofAlzheimer's disease (AD) to improve cognitive function. These enzyme inhibitors were reported to be found in manyplants. Apai-sa-le recipe was a Thai tradition used as nootropic recipe and formerly claimed to improve memory. Therefore, it is interesting to investigate cholinesterase inhibitory activity ofthe recipe and its ingredients.
To determine the whole recipe ofApai-sa-le and its ingredients for inhibitory effect on acetylcholinesterase (AChE) and human butyrylcholinesterase (BuChE) activities.
METHODS AND RESULTS:
Thirty grams of each plant and 181 grams of the whole recipe were separately extracted by 95% ethanol, after filtered the filtrate were evaporated and vacuum-dried at 45°C. By Elman method, the inhibitory activities of both enzymes were assessed. The volatile constituents ofeach extract were determined by GCMS. The constituents in the non- volatile extract were examined by TLC and the antioxidant activity was determined.
Four plants exhibited specific BuChE inhibitor were Lepidium sativum Linn. (Ls), Piper nigrum L. (Pn), Angelica dahurica Benth (Ad) andAtractylodes lancea DC. (Al), which shown the lC50 of 5.59, 24.52, 73.23, 96.25 μg/ml, respectively whereas galantamine and the whole recipe showed IC50 of 0.59 and 236 μg/ml. Only Pn extract inhibited AChE at lCso of 25.46 μg/ml. By GCMS and TLC fingerprints revealed the main constituents in LS, Ad, Al andPn as apiol, cumialdehyde, Furanodiene and piperine. Moreover nine plant extracts and the whole recipe showed antioxidant activity.
CONCLUSIONS:
Lepidium sativum Linn. (Ls) extract showed the most potency on BuChE inhibitory effect. Three ingredients and the whole recipe exhibited mild activity. Only Piper nigrum L demonstrated inhibition effect on both AChE and BuChE.
Bioorg Med Chem Lett. 2017 Dec 13. pii: S0960-894X(17)31189-7.
Cytotoxicity and inhibition of leukemic cell proliferation by sesquiterpenes from rhizomes of Mah-Lueang (Curcuma cf. viridiflora Roxb.).[Pubmed:
29274817]
Curcuma cf. viridiflora Roxb., also known as Mah-Lueang in Thai, belongs to the Zingiberaceae family and is grown from rhizomes. The rhizome of the plant has been used for medicinal purposes, in particular, to treat paralysis in Thai traditional medicine. However, no biologically active compounds have been reported from Mah-Lueang yet.
METHODS AND RESULTS:
In this study, natural compounds were isolated from Mah-Lueang and structurally determined by spectroscopic methods, including electrospray ionization mass spectrometry and nuclear magnetic resonance. The four isolated compounds were identified as Furanodiene (1), dehydrocurdione (2), germacrone-4,5-epoxide (3), and zedoarondiol (4). These sesquiterpenes were investigated for antileukemic activities against KG1a and Molt4 cells. Leukemic cell proliferation is regulated by the Wilms' tumor 1 (WT1) transcription factor. Compound 1 showed the strongest cytotoxicity against both KG1a and Molt4 cells. Noncytotoxic concentrations (20% inhibitory concentration values) of all compounds were able to decrease the WT1 protein expression and total cell numbers in both cell lines. The four compounds showed good inhibitory activities for WT1 protein expression. Compounds 3 and 4 showed excellent antileukemic activities for both cell lines.
CONCLUSIONS:
In summary, four sesquiterpene compounds with antileukemic activities against the KG1a and Molt4 cell lines were identified in Mah-Lueang extracts.