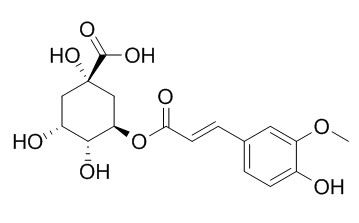
3-O-Feruloylquinic acid
3-O-Feruloylquinic acid is a protease inhibitor, it exerts moderate inhibitory effect against AIV (H5N1) in vitro.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2021, 22(19):10220.
J Med Food.2020, 23(6):633-640.
Pathol Res Pract.2024, :260:155445.
J Biomol Struct Dyn.2023, 1-21.
Journal of Medicinal Food2023, Vol.26(10).
Food Res Int.2024, 197(Pt 1):115244.
Int J Anal Chem.2017, 2017:1254721
Int J Mol Sci.2023, 25(1):162.
J of the Korean Society of Food Science and Nutrition2019, 32(2):148-154
Biomed Pharmacother.2024, 179:117346.
Related and Featured Products
Food Funct. 2013 Nov;4(11):1632-8.
Isolation and quantification of major chlorogenic acids in three major instant coffee brands and their potential effects on H2O2-induced mitochondrial membrane depolarization and apoptosis in PC-12 cells.[Pubmed:
24061869]
Coffee is a most consumed drink worldwide, with potential health effects on several chronic diseases including neuronal degenerative diseases. Chlorogenic acids (CHAs) are phenolic compounds found in coffee and they are reported to have strong antioxidant and anti-inflammatory activities. However, the amounts of CHAs often vary in coffee drinks and their potential effects on ROS-induced neuronal cell death still require more investigation.
METHODS AND RESULTS:
Therefore, in this paper, major CHAs were isolated from three major instant coffee brands, confirmed and quantified using HPLC and NMR spectroscopic methods. Then, their antioxidant activities and protective effects on H2O2-induced apoptosis in PC-12 cells were investigated using radical scavenging, mitochondrial membrane potential and caspase assays. In the coffee samples, three major CHAs (3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid) and some minor CHAs (3-O-Feruloylquinic acid, 4-O-feruloylquinic acid, 5-O-feruloylquinic acid, 3,5-O-dicaffeoylquinic acid, 3,4-O-dicaffeoylquinic acid, and 4,5-O-dicaffeoylquinic acid) were detected. The three major CHAs were further isolated and their chemical structures were confirmed using NMR spectroscopic techniques. Also, the amounts of the three major CHAs were individually quantified using a HPLC method. At the concentration of 10 μM, all three major CHAs quenched DPPH and/or xanthine oxidase-generated radical species by 21-51% (P < 0.014). They also inhibited H2O2-induced mitochondrial membrane depolarization and caspase-9 activation by 27% (P < 0.034) and 50% (P < 0.05), respectively.
CONCLUSIONS:
This study suggests that the major CHAs found in coffee are likely to be potent antioxidant compounds able to quench radical species as well as inhibit H2O2-induced apoptosis via suppressing mitochondrial membrane depolarization and caspase-9 activation in the cells.
Yao Xue Xue Bao. 2015 Feb;50(2):207-10.
Chemical constituents of the roots of Macleaya microcarpa and activation efficacy of benzophenanthridine alkaloids for the transcription of xbp1 gene.[Pubmed:
25975030]
Ongoing study on the chemical constituents of the roots of Macleaya microcarpa led to the isolation of eight compounds of derivatives of triterpenes and organic acids in addition to some previously identified benzophenanthridines.
METHODS AND RESULTS:
The eight compounds were identified by spectroscopic methods as well as comparison with literature values as 1-oxo-2, 22 (30)-hopandien-29-oic acid (1), 3-oxo-12-oleanen-30-oic acid (2), 3α-hydroxy-12-oleanen-30-oic acid (3), 3β-hydroxy-12-oleanen-30-oic acid (4), ferulic acid (5), ferulic acid 4-O-β-D-glucoside (6), 3-O-Feruloylquinic acid (7), and methyl 3-O-feruloylquinate (8). Of which, 1 is a new triterpenoid of hopanes and 2-8 are isolated from M microcarpa for the first time. In order to discover natural active compounds as potential agents of anti-ulcerative colitis (UC), an in vitro drug high-throughput screening model targeted x-box-binding protein 1 (xbp1) was employed to evaluate the activity of the major chemical constituents of M microcarpa.
CONCLUSIONS:
The result confirmed that two dihydrobenzophenanthridines, dihydrosanguinarine (9) and dihydrochelerythrine (10), showed a certain activity on activating the transcription of xbpl, a transcription factor (TF) associated with the occurrence, development, and potential treatment of UC, with their relative activating ratios being 1.76 and 1.77 times, respectively, as compared with control group.