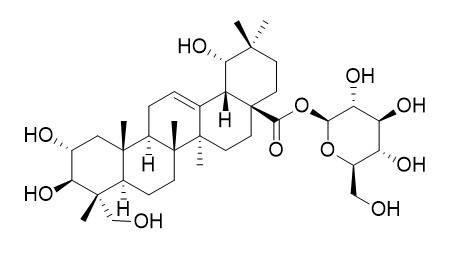
Arjunglucoside I
Arjunglucoside I has anti-inflammatory activity, it exhibits significant activity against carrageenan-induced paw edema in rat. It also shows antiproliferative activity against the A2780 human ovarian cancer cell line with an IC(50) value of 1.2 microM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Viruses.2024, 16(7):1128.
J Cell Biochem.2024, 125(4):e30537.
Front Pharmacol.2021, 12:764297.
Foods.2022, 11(12):1708.
Appl. Sci. 2021, 11(23),11099.
Food and Chemical Toxicology2020, 111221
Kor. J. Herbol.2022, 37(5): 89-96.
Korean J. Agricultural Science2024, 51(3).
Chem Biol Interact.2016, 260:168-175
Food Chem.2020, 313:126079
Related and Featured Products
Phytochemistry. 2010 Jan;71(1):95-9.
Saponins and a lignan derivative of Terminalia tropophylla from the Madagascar Dry Forest.[Pubmed:
19875137]
A study of an EtOH extract obtained from the roots of the Madagascan plant Terminalia tropophylla H. Perrier (Combretaceae) led to isolation of the oleanane-type triterpenoid saponin 1, the lignan derivative 2, and the two known saponins Arjunglucoside I (3) and sericoside (4).
METHODS AND RESULTS:
The structures of compounds 1 (terminaliaside A) and 2 (4'-O-cinnamoyl cleomiscosin A) were elucidated using 1D and 2D NMR experiments and mass spectrometry. Compound 1 showed antiproliferative activity against the A2780 human ovarian cancer cell line with an IC(50) value of 1.2 microM.
Phytochemistry Letters, 2008, 1(4):183-187.
Polyhydroxyoleanane-type triterpenoids from Combretum molle and their anti-inflammatory activity.[Reference:
WebLink]
A new oleanane-type triterpene saponin, β-d-glucopyranosyl 2α,3β,6β-trihydroxy-23-galloylolean-12-en-28-oate (1), together with four known oleanane-type pentacyclic triterpenoids, combregenin (2), arjungenin (3), Arjunglucoside I (4), and combreglucoside (5) were isolated from the stem bark of Combretum molle.
METHODS AND RESULTS:
Their structures were established mainly on the basis of 1D and 2D NMR spectral data. Compounds 1–3 exhibited more significant activity against carrageenan-induced paw edema in rat compared to compounds 4 and 5.Graphical abstractThe new triterpene saponin, β-d-glucopyranosyl 2α,3β,6β-trihydroxy-23-galloylolean-12-en-28-oate (1) together with the known combregenin (2), arjungenin (3), Arjunglucoside I (4), and combreglucoside (5) were isolated from the stem bark of Combretum molle.
CONCLUSIONS:
Their structures were identified by interpretation of their spectral data, mainly ESIMS, 1D NMR (1H, 13C NMR, DEPT) and 2D NMR (COSY, HSQC and HMBC), and by comparison with the literature. Compounds 1–3 exhibited more significant anti-inflammatory activity against carrageenan-induced paw edema in rat compared to compounds 4 and 5.
Z Naturforsch C. 2017 May 1;72(5-6):203-208.
Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential.[Pubmed:
27997356]
METHODS AND RESULTS:
Termiglaucescin (1), a new triterpene glucoside, has been isolated from the ethyl acetate extract of the root bark of Terminalia glaucescens Planch. ex Benth, together with 11 known compounds, β-D-glucopyranosyl 2α,3β,6β-trihydroxy-23-galloylolean-12-en-28-oate (2), Arjunglucoside I (3), sericoside (4), arjungenin (5), sericic acid (6), arjunetin (7), chebuloside II (8), 3,3',4-tri-O-methylelagic acid (9), 3,3'-di-O-methylelagic acid (10), β-sitosterol (11) and stigmasterol (12). Compounds 2, 3, 7, 8 and 9 are reported from the plant for the first time.
CONCLUSIONS:
The structures of the isolated compounds were characterized by spectroscopic data interpretations, especially 1D and 2D NMR. The triterpenic isolates showed potent antioxidant and anti-inflammatory activities.