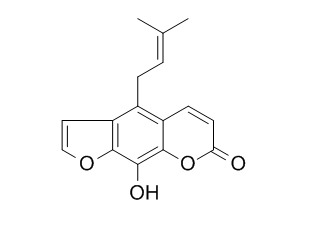
Alloimperatorin
Alloimperatorin exhibits in vitro antitumor activity in HL-60 acute myeloid leukemia cancer cells via inducing apoptosis, cell cycle disruption and inhibition of cell migration. It photosensitizes efficiently the hemolysis of erythrocytes.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Agric Food Chem.2020, 68(43):12164-12172.
Evid Based Complement Alternat Med.2020, 2020:9416962.
J Biomol Struct Dyn.2022, 5;1-17.
Plant Archives2020, 2(1),2929-2934
Oncol Lett.2020, 20(4):122.
Int J Mol Sci.2015, 16(8):18396-411
J Pharmaceut Biomed2020, 178:112894
J Ethnopharmacol.2017, 206:327-336
Int J Mol Sci.2020, 21(19):7209.
Natural Product Sciences2024, 30(4):254-261.
Related and Featured Products
Bangladesh Journal of Pharmacology, 2016, 11(1):194.
Alloimperatorin and its epoxide derivative exhibit in vitro antitumor activity in HL-60 acute myeloid leukemia cancer cells via inducing apoptosis, cell cycle disruption and inhibition of cell migration[Reference:
WebLink]
The aim of the present study was to synthesize epoxide derivative of Alloimperatorin and evaluating its antitumor and apoptotic effects in acute myeloid leukemia HL-60 cells.
METHODS AND RESULTS:
The cytotoxic effects were demonstrated by MTT assay. Fluorescence microscopy along with flow cytometry were performed to evaluate the effect of Alloimperatorin epoxide on apoptosis and cell cycle. In vitro wound healing assay was performed to study compounds effect on cancer cell migration.
METHODS AND RESULTS:
The results indicated that Alloimperatorin epoxide (IC50 = 32.1 μM) was much more effective in inhibiting HL-60 cancer cell growth as compared to Alloimperatorin (IC50 = 128 μM). Further, Alloimperatorin epoxide induced apoptosis related morphological alterations in HL-60 cells including blebbing of plasma membrane, DNA fragmentation and formation of apoptotic bodies.
Alloimperatorin epoxide also led to G2/M phase cell cycle arrest and suppressed HL-60 cancer cell migration indicating that this compound may be a promising candidate for the treatment of cancer metastasis.
Photochemistry & Photobiology, 2014, 90(1):162-170.
Photohemolysis Sensitized by the Furocoumarin Derivative Alloimperatorin and its Hydroperoxide Photooxidation Product.[Pubmed:
24117477]
METHODS AND RESULTS:
The dark and photosensitized effects of Alloimperatorin methyl ether 1 (hereafter simply Alloimperatorin) and its photooxygenation product Alloimperatorin hydroperoxide 2 were investigated on human erythrocytes. The results reveal that the furocoumarin 1 photosensitizes efficiently the hemolysis of erythrocytes. The rate of photohemolysis increases on raising the temperature of the postirradiated incubation from 4°C to 37°C. Thermal activation of the photohemolysis and inhibition by 2,6-di-tert-butyl-p-cresol (BHT) suggest that the furocoumarin 1 photosensitizes lipid peroxidation, increasing permeability in the erythrocyte membrane. The hydroperoxide 2 induces dark and photosensitized hemolysis more efficiently than the furocoumarin 1. The rate of hemolysis induced by 2 increases with the incubation temperature and decreases in the presence of tert-butanol and BHT. The hydroperoxide 2 photosensitizes the formation of lipid peroxidation products as shown by the reaction with thiobarbituric acid. This process is diminished by BHT.
CONCLUSIONS:
Our data imply that the photohemolysis sensitized by the furocoumarin 1 is caused by the in situ-formed photooxygenation product 2. Such hydroperoxides are potent hemolytic agents in the dark and especially on photosensitization.