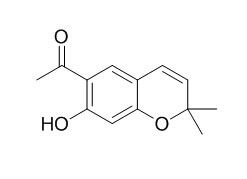
Eupatoriochromene
Eupatoriochromene retard seed germination , reduced radicle and hypocotyl growth of weed and crop plant seedlings and increased adventitious root formation of mung bean cuttings. Eupatoriochromene has insecticidal activity, it exhibits toxicity againstCulex pipiens (house mosquito) larvae andOncopeltus fasciatus (large milkweed bug) nymphs.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Cell Biochem.2024, 125(4):e30537.
Horticulturae2024, 10(4), 382.
Food Funct.2023, 14(9):4354-4367.
Evid Based Complement Alternat Med.2015, 2015:165457
Appl Biol Chem2019, 62:46
In Vitro Cellular & Developmental Biology - Plant2022, 58:972-988.
J Ethnopharmacol.2024, 326:117902.
Foods.2023, 12(2):318.
Plant Cell Physiol.2018, 59(1):128-141
Chem Biodivers.2023, 20(10):e202300741.
Related and Featured Products
J Chem Ecol. 1989 Jul;15(7):2073-87.
Eupatoriochromene and encecalin, plant growth regulators from yellow starthistle (Centaurea solstitialis L.).[Pubmed:
24272297]
METHODS AND RESULTS:
Two chromenes, Eupatoriochromene (1) and encecalin (2), have been isolated from yellow starthistle (Centaurea solstitialis L.). Both chromenes retard seed germination and reduce radicle and hypocotyl growth of weed and crop plant seedlings. In addition,1 increases adventitious root formation of mung bean cuttings.
J Chem Ecol. 1985 Jun;11(6):701-12.
Insecticidal chromenes from the volatile oil ofHemizonia fitchii.[Pubmed:
24310216]
Based on field observations of the effects of the resinous tarweedHemizonia fitchii A. Gray (Asteraceae) on mosquito populations in California, the volatile oil of this plant was investigated for insecticidal activity.
METHODS AND RESULTS:
Analysis of thé oil by TLC and capillary GC-MS showed the presence of five major constituentś which were identified as the monoterpenoid 1,8-cineole, and the chromenes encecalin, Eupatoriochromene (desmethylencecalin), 6-vinyl-7-methoxy-2,2-dimethylchromene, and desmethoxyencecalin. Trace amounts of several volatile fatty acids, alkanes,p-coumarate derivatives, additional chromene derivatives, and numerous mono- and sesquiterpenoids were also detected and identified by GC-MS. Fractionation of the oil by preparative TLC and column chromatography afforded the major chromenes, the identities of which were confirmed by NMR and IR spectral data.
CONCLUSIONS:
The chromenes exhibited weak to moderate toxicity againstCulex pipiens (house mosquito) larvae andOncopeltus fasciatus (large milkweed bug) nymphs. However, no antijuvenile hormone activity was observed for any of the compounds tested against these insect species.