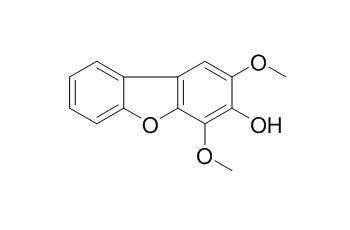
Eriobofuran
Eriobofuran, a phytoalexin, has antimicrobialactivity, it can inhibit strongly the spore germination and germ tube growth of Pestalotia funerea, a host-pathogenic fungus of loquat tree.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chemistry of Natural Compounds2019, 55(1):127-130
Pharmaceuticals (Basel).2022, 15(5):591.
Antioxidants (Basel).2021, 10(10):1620.
Molecules.2023, 28(16):6025.
Antioxidants (Basel).2021, 10(11):1831.
J Applied Biological Chemistry2021, 64(2):185-192
Molecules.2020, 25(18):4283.
Plants (Basel).2023, 12(1):163.
JPC-Journal of Planar Chromatography2023, 36:179-190
Molecules.2022, 27(21):7643.
Related and Featured Products
Montixanthone
Catalog No: CFN91903
CAS No: 876305-36-1
Price: Inquiry(manager@chemfaces.com)
3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No: CFN89303
CAS No: 262292-34-2
Price: Inquiry(manager@chemfaces.com)
Onjixanthone II
Catalog No: CFN89276
CAS No: 136083-93-7
Price: Inquiry(manager@chemfaces.com)
6-Hydroxy-1,2,3,7-tetramethoxyxanthone
Catalog No: CFN89282
CAS No: 64756-87-2
Price: Inquiry(manager@chemfaces.com)
1,2,3,6,7-Pentamethoxyxanthone
Catalog No: CFN89241
CAS No: 64756-86-1
Price: Inquiry(manager@chemfaces.com)
Angustin A
Catalog No: CFN89373
CAS No: 1415795-50-4
Price: Inquiry(manager@chemfaces.com)
Angustin B
Catalog No: CFN89374
CAS No: 1415795-51-5
Price: Inquiry(manager@chemfaces.com)
7,8,9-Trimethoxy-10H-1,3-dioxolo[4,5-b]xanthen-10-one
Catalog No: CFN98533
CAS No: 24562-58-1
Price: Inquiry(manager@chemfaces.com)
1,5,6-Trihydroxy-3,7-dimethoxyxanthone
Catalog No: CFN89047
CAS No: 65008-02-8
Price: Inquiry(manager@chemfaces.com)
1,5,6-Trihydroxyxanthone
Catalog No: CFN89364
CAS No: 5042-03-5
Price: Inquiry(manager@chemfaces.com)
Journal of Pesticide Science, 1985, 10(1):101-106.
Isolation and Structural Determination of Eriobofuran, A New Dibenzofuran Phytoalexin from Leaves of Loquat, Eriobotrya japonica L[Reference:
WebLink]
METHODS AND RESULTS:
An antimicrobial substance, named as Eriobofuran (Ia) was isolated from loquat leaves [Eriobotrya japonica L. (Rosaceae)] inoculated with Entomosporium eriobotryae, a host-pathogenic fungus. The structure of Eriobofuran was deduced to be 2, 4-dimethoxy-3-hydroxy-dibenzofuran by spectroscopic, chemical evidence and biosynthetic considerations. Its structure was confirmed by direct comparison with a synthetic specimen of Eriobofuran ethyl ether.
CONCLUSIONS:
Eriobofuran inhibited strongly the spore germination and germ tube growth of Pestalotia funerea, a host-pathogenic fungus of loquat tree.
Zhong Yao Cai. 2012 Mar;35(3):396-9.
Chemical constituents from the fruits of Kadsura marmorata.[Pubmed:
22876677]
To study the chemical constituents from the fruits of Kadsura marmorata.
METHODS AND RESULTS:
The chemical constituents were isolated and purified by chromatography on silica gel, Sephadex LH-20 column and HPLC. 9 compounds were isolated and identified as 4,5-dihydroxy-3-methoxybiphenyl (I), Eriobofuran (II), 3beta, 16beta-dihydroxy urs-2-ene (III), 2alpha, 3beta, 6beta, 23-tetrahydroxy urs-12,18-dien-28-oic acid (IV), 2alpha,3beta,23-trihydroxy urs-12-en-28-oic acid (V), rutin (VI), 2-ethylhexanoic acid (VII), 2-monolaurin (VIII), glyceryl monoricinoleate (IX) on the basis of NMR and EI-MS spectroscopic analysis.
CONCLUSIONS:
All these compounds are isolated from this genus for the first time.