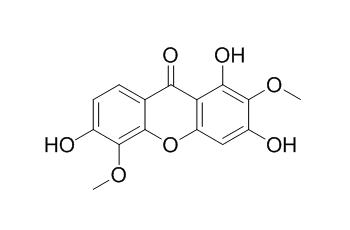
1,3,6-Trihydroxy-2,5-dimethoxyxanthone
1,3,6-Trihydroxy-2,5-dimethoxyxanthone may have antifungal activity. It also shows antimutagenic potential, in particular preventing mutations caused by aflatoxin B1 (AFB1) and benzo[a]pyrene (B[a]P).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chinese Pharmacological Bulletin2019, 35(8):1120-1125
Heliyon.2024, 10(7):e28755.
J Ethnopharmacol.2018, 210:88-94
Acta Physiologiae Plantarum2015, 37:1736
Korean J. Medicinal Crop Sci.2023, 31(6):388-395.
Kor.J.Herbol.2024, 39(3):23-35
Biochem Biophys Res Commun.2019, 518(4):732-738
J Chromatogr A.2017, 1518:46-58
Methods Protoc.2024, 7(6):95.
Cell J.2024, 26(8):496-504.
Related and Featured Products
Bellidifolin
Catalog No: CFN89132
CAS No: 2798-25-6
Price: $268/10mg
Gentisein
Catalog No: CFN92398
CAS No: 529-49-7
Price: Inquiry(manager@chemfaces.com)
1,3,5-Trihydroxyxanthone
Catalog No: CFN92399
CAS No: 6732-85-0
Price: Inquiry(manager@chemfaces.com)
1,3,5,6-Tetrahydroxyxanthone
Catalog No: CFN92539
CAS No: 5084-31-1
Price: Inquiry(manager@chemfaces.com)
1,3,6-Trihydroxy-5-methoxyxanthone
Catalog No: CFN92540
CAS No: 41357-84-0
Price: Inquiry(manager@chemfaces.com)
3,8-Dihydroxy-2,4,6-trimethoxyxanthone
Catalog No: CFN92556
CAS No: 65008-17-5
Price: Inquiry(manager@chemfaces.com)
1,6,7-Trihydroxyxanthone
Catalog No: CFN98284
CAS No: 25577-04-2
Price: Inquiry(manager@chemfaces.com)
Norathyriol
Catalog No: CFN98468
CAS No: 3542-72-1
Price: $368/5mg
Montixanthone
Catalog No: CFN91903
CAS No: 876305-36-1
Price: Inquiry(manager@chemfaces.com)
3,6-Dihydroxy-1,7-dimethoxyxanthone
Catalog No: CFN89303
CAS No: 262292-34-2
Price: Inquiry(manager@chemfaces.com)
Steroids. 2013 Nov;78(11):1053-63.
Estrogenic and chemopreventive activities of xanthones and flavones of Syngonanthus (Eriocaulaceae).[Pubmed:
23891669]
METHODS AND RESULTS:
We used recombinant yeast assay (RYA), with the strain BY4741 of Saccharomyces cerevisiae, and Ames test, with strains TA100, TA98, TA97a and TA102 of Salmonella typhimirium, to evaluate estrogenicity, mutagenicity and antimutagenicity of methanolic extracts of Syngonanthus dealbatus (S.d.), Syngonanthus macrolepsis (S.m.), Syngonanthus nitens (S.n.) and Syngonanthus suberosus (S.s.), and of 9 compounds isolated from them (1=luteolin, 2=mix of A-1,3,6-trihydroxy-2-methoxyxanthone and B-1,3,6-Trihydroxy-2,5-dimethoxyxanthone, 3=1,5,7-trihydroxy-3,6-dimethoxyxanthone, 4=1,3,6,8-tetrahydroxy-2,5-dimethoxyxanthone, 5=1,3,6,8-tetrahydroxy-5-methoxyxanthone, 6=7-methoxyluteolin-8-C-β-glucopyranoside, 7=7-methoxyluteolin-6-C-β-glucopyranoside, 8=7,3'-dimethoxyluteolin-6-C-β-glucopyranoside and 9=6-hydroxyluteolin). The results indicated the estrogenic potential of the S. nitens methanol extract and four of its isolated xanthones, which exhibited, respectively, 14.74±1.63 nM; 19.54±6.61; 7.20±0.37; 6.71±1.02 e 10.01±4.26 nM of estradiol-equivalents (EEQ). None of the extracts or isolated compounds showed mutagenicity in any of the test strains and all of them showed antimutagenic potential, in particular preventing mutations caused by aflatoxin B1 (AFB1) and benzo[a]pyrene (B[a]P).
CONCLUSIONS:
The results show that the xanthones, only isolated from the methanol extract of S. nitens capitula, probably were the responsible for its estrogenic activity and could be useful as phytoestrogens, providing a new opportunity to develop hormonal agents. In addition, flavones and xanthones could also be used as a new antimutagenic agent. Since, the mutagens are involved in the initiation and promotion of several human diseases, including cancer, the significance of novel bioactive phytocompounds in counteracting these pro-mutagenic and carcinogenic effects is now gaining credence.
J Nat Prod. 2004 Sep;67(9):1450-4.
Lipoxygenase inhibitory constituents from Periploca aphylla.[Pubmed:
15387640]
METHODS AND RESULTS:
Bisflavan-3-ols 1 and 2 and norterpenoid 3 have been isolated from the methanolic extract of the whole plant of Periploca aphylla. Their structures have been assigned on the basis of spectroscopic analysis including 1D and 2D NMR techniques. In addition, o-phthalic acid bis(2-ethylnonyl) ester (4), 1,3,6-Trihydroxy-2,5-dimethoxyxanthone (5), and (+)-lyoniresinol (6) have been reported for the first time from this species.
CONCLUSIONS:
Compounds 1-3 displayed evident inhibitory potential against the enzyme lipoxygenase in a concentration-dependent fashion with IC(50) values 19.7, 13.5, and 150.1 microM, respectively.
Phytochemistry. 1994 Oct;37(3):875-8.
Xanthone and antifungal constituents from Monnina obtusifolia.[Pubmed:
7765695]
METHODS AND RESULTS:
Three biphenyls and four xanthones have been isolated from the aerial parts of Monnina obtusifolia. The structures were established on the basis of their spectral data and that of some derivatives. The biphenyls have been isolated previously from the same genus. 1,3,6-Trihydroxy-2,5-dimethoxyxanthone is a new natural product, whereas the other xanthones have been described in other species.
CONCLUSIONS:
The antifungal activity of the isolated compounds has been determined.