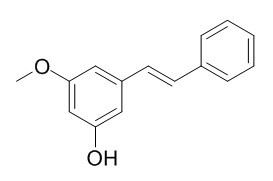
Pinosylvin monomethyl ether
Pinosylvin monomethyl ether (E)-3-Hydroxy-5-methoxystilbene possesses inhibitory activity against several Gram-positive bacteria, including isolates of methicillin-resistant Staphylococcus aureus (MRSA), Mycobacterium bovis BCG, and avirulent Bacillusanthracis (Sterne strain), among others.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Cell.2017, 68(4):673-685
Metabolites2022, 12(6),507.
Food Research International2020, 108987
J.Korean Society of Grassland&Forage Science2023, 43(3):138-147.
Iranian J. Pharm. Res.2021, 20(4):59-70
Prev Nutr Food Sci.2024, 29(4):504-511.
Turkish Journal of Pharmaceutical Sciences2022, DOI: 10.4274
Food Funct.2022, 13(23):12105-12120.
Asian J Beauty Cosmetol2020, 18(3): 265-272.
Phytother Res.2015, 29(7):1088-96
Related and Featured Products
Bioorg Med Chem Lett. 2008 Nov 1;18(21):5745-9.
New classes of Gram-positive selective antibacterials: inhibitors of MRSA and surrogates of the causative agents of anthrax and tuberculosis.[Pubmed:
18849164]
METHODS AND RESULTS:
An antimicrobial phenolic stilbene, (E)-3-Hydroxy-5-methoxystilbene, 1 was recently isolated from the leaves of Comptonia peregrina (L.) Coulter and shown to possess inhibitory activity against several Gram-positive bacteria, including isolates of methicillin-resistant Staphylococcus aureus (MRSA), Mycobacterium bovis BCG, and avirulent Bacillusanthracis (Sterne strain), among others. These results prompted the design and synthesis of two new classes of compounds, phenoxystyrenes and phenothiostyrenes, as analogs of the natural antimicrobial stilbene. These and additional stilbenoid analogs were synthesized using new, efficient, copper-mediated coupling strategies. Minimum inhibitory concentration (MIC) antimicrobial assays were performed on all compounds prepared.
CONCLUSIONS:
These preliminary structure-activity relationship studies indicated that both new classes of synthetic analogs, as well as the stilbenes, show promising activity against Gram-positive bacteria when at least one phenolic moiety is present, but not when absent. The potencies of the phenolic phenoxystyrenes and phenothiostyrenes were found to be comparable to those of the phenolic stilbenes tested.