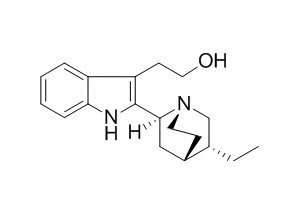
Dihydrocinchonamine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biochem Biophys Res Commun.2017, 482(4):1095-1101
LWT-Food Sci Technol2020, 109163
Horticulturae2020, 6(4),76.
Free Radic Biol Med.2017, 112:191-199
Br J Pharmacol.2020, 10.1111
Acta Agriculturae Scandinavica2015, 381-383
Nutr Cancer.2022, 1-13.
Int J Food Sci Nutr.2019, 70(7):825-833
Front Immunol.2018, 9:2091
J Med Chem.2023, 66(6):4106-4130.
Related and Featured Products
The Alkaloids: Chemistry and Physiology Volume 11, 1968, Pages 73–77
Chapter 3 The 2,2′-Indolylquinuclidine Alkaloids[Reference:
WebLink]
The group of 2,2′-indolylquinuclidine alkaloids has a narrow distribution and only two members have been discovered in the past few years. This chapter focuses on accepted stereochemistry, although an unequivocal proof is still required for C-3 in cinchonamine.
METHODS AND RESULTS:
The course of the acetylation of cinchonamine has been restudied using the parent base, Dihydrocinchonamine and O-tritylcinchonamine. Many of the compounds formed in this investigation were amorphous, but were satisfactorily characterized via thin-layer chromatography and mass spectrometry, along with UV- and IR-measurements. At low temperatures (dry ice-acetone), cinchonamine yields O-acetylcinchonamine. Dihydrocinchonamine behaves analogously upon treatment with acetic anhydride, although there are difficulties because of the amorphous nature of the products.
CONCLUSIONS:
Cinchophyllamine contains two 5-methoxyindole nuclei with unsubstituted nitrogen atoms, a vinyl group, and two basic nitrogens of which one is tertiary and the other secondary. A reinvestigation of the alkaloid content of the leaves of Cinchona legeriana Moens gives, besides quinamine, two new bases analyzing for C31H36N4O2—cinchophyllamine and isocinchophyllamine.