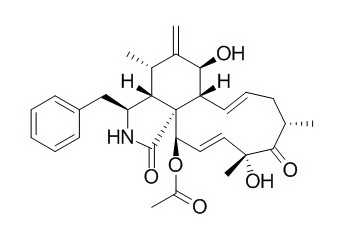
Cytochalasin D
Cytochalasin D is an actin inhibitor, the removal of actin stress fibers is crucial for the chondrogenic differentiation. It may be an inhibitor of some fertilization processes such as sperm penetration or sperm head decondensation. Cytochalasin D inhibits CT26 tumor growth potentially through inhibition of cell proliferation, induction of cell apoptosis and suppression of tumor angiogenesis; it stimulates the expression of TF in B16 melanoma cells, activating both coagulation-dependent and -independent pathways via binding to FVIIa, eventually promoting lung metastasis. Cytochalasin D is also an inhibitor of microfilament-dependent phagocytosis, it (0.5 or 1.0 micrograms/ml) can inhibit intracellular multiplication of L. pneumophila in U937 monocytes. Cytochalasin D inhibits smooth muscle contraction by directly inhibiting contractile apparatus.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plant Biotechnology Reports 2021, 15:117-124.
Plant Cell Physiol.2018, 59(1):128-141
Cancer Lett. 2023, 18:216584.
Mol Cancer Ther.2024, 1535-7163.
Neurotox Res.2020, 38(1):163-174.
Journal of Applied Biology & Biotechnology2023,11(4):148-158
J Med Food.2024, 27(9):844-856.
EXCLI J.2023, 22:482-498.
Indian J Pharm Sci.2024, 86(2):736-741.
J Sep Sci.2018, 41(11):2488-2497
Related and Featured Products
Cytometry A. 2013 Sep;83(9):830-8.
Inhibition of cytoplasmic streaming by cytochalasin D is superior to paraformaldehyde fixation for measuring FRET between fluorescent protein-tagged Golgi components.[Pubmed:
23520174]
Protein-protein interaction at the organelle level can be analyzed by using tagged proteins and assessing Förster resonance energy transfer (FRET) between fluorescent donor and acceptor proteins. Such studies are able to uncover partners in the regulation of proteins and enzymes. However, any organelle movement is an issue for live FRET microscopy, as the observed organelle must not change position during measurement. One of the mobile organelles in plants is the Golgi apparatus following cytoplasmic streaming. It is involved in the decoration of proteins and processing of complex glycan structures for the cell wall. Understanding of these processes is still limited, but evidence is emerging that protein-protein interaction plays a key role in the function of this organelle. In the past, mobile organelles were usually immobilized with paraformaldehyde (PFA) for FRET-based interaction studies.
METHODS AND RESULTS:
Here, we show that the actin inhibitor Cytochalasin D (CytD) is superior to PFA for immobilization of Golgi stacks in plant cells. Two glycosyltransferases known to interact were tagged with cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP), respectively, coexpressed in Nicotiana benthamiana leaves and analyzed using confocal microscopy and spectral imaging. Fixation with PFA leads to reduced emission intensity when compared to CytD treatment. Furthermore, the calculated FRET efficiency was significantly higher with CytD than with PFA.
CONCLUSIONS:
The documented improvements are beneficial for all methods measuring FRET, where immobilization of the investigated molecules is necessary. It can be expected that FRET measurement in organelles of animal cells will also benefit from the use of inhibitors acting on the cytoskeleton.
Asian Pac J Trop Med. 2012 Mar;5(3):169-74.
Cytochalasin D, a tropical fungal metabolite, inhibits CT26 tumor growth and angiogenesis.[Pubmed:
22305779]
To investigate whether Cytochalasin D can induce antitumor activities in a tumor model.
METHODS AND RESULTS:
Murine CT26 colorectal carcinoma cells were cultured in vitro and Cytochalasin D was used as a cytotoxic agent to detect its capabilities of inhibiting CT26 cell proliferation and inducing cell apoptosis by MTT and a TUNEL-based apoptosis assay. Cytochalasin D inhibited CT26 tumor cell proliferation in time and dose dependent manner and induced significant CT26 cell apoptosis, which almost reached the level induced by the positive control nuclease. The optimum effective dose of Cytochalasin D for in vivo therapy was about 50 mg/kg. Cytochalasin D in vivo treatment significantly inhibited tumor growth and prolonged the survival times in CT26 tumor-bearing mice. The results of immunohistochemistry analysis and alginate encapsulation assay indicated that the Cytochalasin D could effectively inhibited tumor angiogenesis.
CONCLUSIONS:
Cytochalasin D inhibits CT26 tumor growth potentially through inhibition of cell proliferation, induction of cell apoptosis and suppression of tumor angiogenesis.
Infect Immun. 1991 Mar;59(3):758-63.
Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells.[Pubmed:
1997428 ]
A cloned and axenically cultured strain of Hartmannella vermiformis was used as a model to study intracellular multiplication of Legionella pneumophila in amoebae.
METHODS AND RESULTS:
The growth of L. pneumophilia in both H. vermiformis and a human monocyte-like cell line (U937) was investigated with cytoskeletal and metabolic inhibitors. L. pneumophila replicated only intracellularly in these cellular models, and electron microscopy showed ultrastructural similarities in the initial phase of multiplication. Treatment of amoebae with an inhibitor of microfilament-dependent phagocytosis (Cytochalasin D, 0.5 or 1.0 micrograms/ml) did not inhibit intracellular growth of L. pneumophila; however, intracellular multiplication was inhibited by treatment of U937 monocytes with the same concentrations of Cytochalasin D. Methylamine (10 to 100 mM), an inhibitor of adsorptive pinocytosis, inhibited the replication of L. pneumophila in amoebae in a dose-dependent manner. All doses of methylamine tested (10 to 50 mM) inhibited growth of L. pneumophila in U937 monocytes. Cytochalasin D and methylamine had no effect on the multiplication of L. pneumophila in culture medium or on the viability of amoebae or U937 monocytes.
CONCLUSIONS:
Intracellular replication of L. pneumophila in H. vermiformis may be accomplished by a Cytochalasin D-independent mechanism, such as adsorptive pinocytosis. In contrast, both Cytochalasin D- and methylamine-sensitive mechanisms may be essential for the intracellular multiplication of L. pneumophila in U937 monocytes.
J Androl. 1989 Jul-Aug;10(4):275-82.
Cytochalasin D inhibits penetration of hamster eggs by guinea pig and human spermatozoa.[Pubmed:
2777719]
METHODS AND RESULTS:
Fertilization experiments using zona-free hamster eggs and spermatozoa from both guinea pig and human were conducted in the presence of Cytochalasin D to evaluate the possible role of actin filaments in fertilization processes. When the actin filament inhibitor, Cytochalasin D, was added to fertilization media at concentrations of 10 to 30 microM, penetration of eggs was significantly inhibited. Preincubation of the eggs with Cytochalasin D and washing prior to addition of spermatozoa had no effect on penetration as quantitated by the number of swollen heads in the egg cytoplasm. However, spermatozoa preincubated with Cytochalasin D and subsequently washed prior to egg addition showed reduced penetration of the same magnitude as when spermatozoa and eggs were coincubated with Cytochalasin D. Both the percentage of zona-free eggs showing decondensed sperm heads and the penetration indices (total decondensed spermatozoa/total eggs) were significantly affected when spermatozoa were exposed to Cytochalasin D. The DMSO vehicle used to dissolve Cytochalasin D had little effect on the number of decondensed heads. When the concentration of Cytochalasin D was increased (DMSO remaining constant) in human sperm experiments, percent penetration decreased and progressively fewer decondensed spermatozoa were recorded, indicating dose-responsiveness to Cytochalasin D. Motility parameters of human spermatozoa were not altered at any of the concentrations of Cytochalasin D tested. Neither guinea pig sperm motility nor acrosome reaction was altered significantly by Cytochalasin D or the DMSO vehicle.
CONCLUSIONS:
These experiments suggest that Cytochalasin D may be an inhibitor of some fertilization processes such as sperm penetration or sperm head decondensation.
J Smooth Muscle Res. 1996 Apr;32(2):51-60.
Cytochalasin D inhibits smooth muscle contraction by directly inhibiting contractile apparatus.[Pubmed:
8845566]
We investigated the mode of relaxant effects of Cytochalasin D, a capping agent of actin filaments, on contractile responses in the rat aorta and chicken gizzard smooth muscles.
METHODS AND RESULTS:
Cytochalasin D inhibited the contraction induced by high K+ or noradrenaline (10 nM-1 microM) without changing cytosolic Ca2+ level ([Ca2+]i) in the rat aorta. In the absence of external Ca2+, 12-deoxyphorbol 13-isobutylate (DPB) (1 microM) induced sustained contraction without increasing in [Ca2+]i and Cytochalasin D also inhibited this contraction. In the permeabilized chicken gizzard smooth muscle, Cytochalasin D inhibited the Ca2+ (1-10 microM)-induced contraction. Cytochalasin D also inhibited the Ca(2+)-independent contraction in the muscle which had been thiophosphorylated by ATP gamma S. Cytochalasin D decreased the velocity of superprecipitation in the chicken gizzard native actomyosin (myosin B) affecting neither the level of MLC phosphorylation nor Mg(2+)-ATPase activity.
CONCLUSIONS:
These results suggest that Cytochalasin D inhibits smooth muscle contractions without any effect on the Ca(2+)-dependent MLC phosphorylation or subsequent activation of myosin ATPase activity. Based on these evidences, it is concluded that Cytochalasin D may inhibit smooth muscle contraction possibly through uncoupling of the force generation from the activated actomyosin Mg(2+)-ATPase.
Exp Mol Med. 2012 Sep 30;44(9):521-8.
Staurosporine and cytochalasin D induce chondrogenesis by regulation of actin dynamics in different way.[Pubmed:
22684244]
Staurosporine and Cytochalasin D modulate actin cytoskeleton and affect chondrogenesis. However, the underlying mechanisms for actin dynamics regulation by these agents are not known well.
METHODS AND RESULTS:
In the present study, we investigate the effect of staurosporine and Cytochalasin D on the actin dynamics as well as possible regulatory mechanisms of actin cytoskeleton modulation. Staurosporine and Cytochalasin D have different effects on actin stress fibers in that staurosporine dissolved actin stress fibers while Cytochalasin D disrupted them in both stress forming cells and stress fiber-formed cells. Increase in the G-/F-actin ratio either by dissolution or disruption of actin stress fiber is critical for the chondrogenic differentiation. Cytochalasin D reduced the phosphorylation of cofilin, whereas staurosporine showed little effect on cofilin phosphorylation. Either staurosporine or Cytochalasin D had little effect on the phosphorylation of myosin light chain.
CONCLUSIONS:
These results suggest that staurosporine and Cytochalasin D employ different mechanisms for the regulation of actin dynamics and provide evidence that removal of actin stress fibers is crucial for the chondrogenic differentiation.
Cell Biol Int. 2012;36(12):1223-31.
Cytochalasin D enhances the accumulation of a protease-resistant form of prion protein in ScN2a cells: involvement of PI3 kinase/Akt signalling pathway.[Pubmed:
22985412]
The conversion of a host-encoded PrPsen (protease-sensitive cellular prion protein) into a PrPres (protease-resistant pathogenic form) is a key process in the pathogenesis of prion diseases, but the intracellular mechanisms underlying PrPres amplification in prion-infected cells remain elusive.
METHODS AND RESULTS:
To assess the role of cytoskeletal proteins in the regulation of PrPres amplification, the effects of cytoskeletal disruptors on PrPres accumulation in ScN2a cells that were persistently infected with the scrapie Chandler strain have been examined. Actin microfilament disruption with Cytochalasin D enhanced PrPres accumulation in ScN2a cells. In contrast, the microtubule-disrupting agents, colchicine, nocodazole and paclitaxel, had no effect on PrPres accumulation. In addition, a PI3K (phosphoinositide 3-kinase) inhibitor, wortmannin and an Akt kinase inhibitor prevented the Cytochalasin D-induced enhancement of PrPres accumulation. Cytochalasin D-induced extension of neurite-like processes might correlate with enhanced accumulation of PrPres.
CONCLUSIONS:
The results suggest that the actin cytoskeleton and PI3K/Akt pathway are involved in the regulation of PrPres accumulation in prion-infected cells.
Oncol Rep. 2013 Jul;30(1):478-84.
Cytochalasin D promotes pulmonary metastasis of B16 melanoma through expression of tissue factor.[Pubmed:
23615686]
Cytochalasin D (CytD) targets actin, a ubiquitous protein in eukaryotic cells. Previous studies have focused mainly on the antitumor effects of Cytochalasin D. We previously found Cytochalasin D to promote lung metastasis in B16 melanoma cells, which we had not anticipated, and, therefore, in the present study we investigated the possible underlying mechanisms. B16 melanoma cells were co-cultured with Cytochalasin D and other agents and used to establish a lung metastatic model.
METHODS AND RESULTS:
In this B16 melanoma metastatic model, significantly increased lung metastasis and lung weight were found in Cytochalasin D-treated mice, which was almost completely suppressed by tissue factor (TF) RNA interference expressed via lentivirus. The results of northern and western blot, and real-time RT-PCR analysis showed that the expression of TF was significantly upregulated in B16 cells treated with Cytochalasin D but was significantly inhibited by TF RNA interference. In addition, upregulation and phosphorylation of mitogen-activated protein kinase p38 were also found in the metastatic lung tissues treated with Cytochalasin D and in the B16 cells co-cultured with Cytochalasin D and factor VIIa (FVIIa), but not in cells cultured with Cytochalasin D, dimethyl sulfoxide or FVIIa alone.
CONCLUSIONS:
These results indicate that Cytochalasin D stimulates the expression of TF in B16 melanoma cells, activating both coagulation-dependent and -independent pathways via binding to FVIIa, eventually promoting lung metastasis.