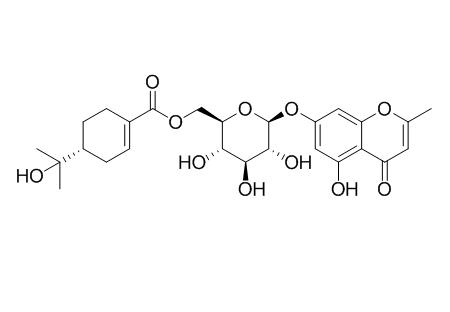
Cypellocarpin C
Cypellocarpin C shows potent in vitro antitumor-promoting activity, it also can suppress an in vivo two-stage carcinogenesis induced with nitric oxide and TPA on mouse skin.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Academic J of Second Military Medical University2018, 39(11)
Nutrients.2019, 12(1)
Food Funct.2024, 15(4):1852-1866.
Sci Rep.2017, 7:467-479
J Food Drug Anal.2023, 31(2):254-277.
Appl. Sci.2020, 10(16),5482.
Legume Science2021, 3(4): e101.
Antioxidants (Basel).2020, 9(6):526.
Int J Pharm.2022, 618:121636.
Korean J. Crop Sci.2018, 63(2):131-139
Related and Featured Products
J Nat Prod. 2000 Sep;63(9):1253-7.
Cypellocarpins A-C, phenol glycosides esterified with oleuropeic acid, from Eucalyptus cypellocarpa.[Pubmed:
11000030]
METHODS AND RESULTS:
Three new phenol glycosides acylated with (+)-oleuropeic acid, cypellocarpin A (1), cypellocarpin B (2), and Cypellocarpin C (3), along with seven known compounds, were isolated from the dried leaves of Eucalyptus cypellocarpa. Structures of the new compounds were determined on the basis of spectroscopic methods, including 2D NMR experiments and chemical degradation.
CONCLUSIONS:
These new compounds and a known related glucoside (7) showed potent in vitro antitumor-promoting activity in a short-term bioassay evaluating the inhibitory effect on Epstein-Barr virus early antigen activation induced by 12-O-tetradecanoyl phorbol 13-acetate (TPA).
These compounds also suppressed an in vivo two-stage carcinogenesis induced with nitric oxide and TPA on mouse skin.
Zhongguo Zhong Yao Za Zhi. 2007 Mar;32(6):496-500.
Studies on chemical constituents in fruits of Eucalyptus globulus.[Pubmed:
17552153]
To study the chemical constituents in the fruits of Eucalyptus globulus Labill.
METHODS AND RESULTS:
The chemical constituents were isolated by various column chromatographic methods and structurally elucidated by IR, NMR and MS evidences. Fifteen compounds were obtained and identified as beta-sitosterol (1), betulinic acid (2), stigmasterol (3), euscaphic acid (4), 2a-Hydroxybetulinic acid (5), macrocarpal B (6), macrocarpal A (7), oleanolic acid (8), 3,4,3'-O-trimethylellagic acid (9), 3-O-methylellagic acid 4'-O-(2"-O-acetyl )-alpha-L-rhamnopyranoside (10), camaldulenside (Cypellocarpin C, 11), 3-O-methylellagic acid 4'-O-alpha-L-rhamnopyranoside (12), 3-O-methylellagic acid (13), ellagic acid (14), and gallic acid (15).
CONCLUSIONS:
Compounds 4 and 5 from genera Eucalyptus, 1, 3 and 11 from plant E. globulus, and 6, 7, 9 and 15 from the fruits of E. globulus were isolated for the first time.