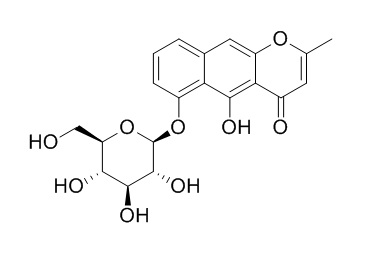
Cassiaside
Cassiaside has significant hepato-protective effects against galactosamine damage, which is higher than that of silybin from Silybum marianum. Cassiaside demonstrates significant antimutagenic, and 1,1-diphenyl-2-picrylhydrazyl(DPPH) radical scavenging effects. Cassiaside and emodi show mixed-type inhibition against β-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
LWT-Food Sci Technol2020, 109163
Int J Mol Sci.2021, 22(8):4211.
Nutrients.2024, 16(19):3266.
J Ethnopharmacol.2023, 321:117501.
Int J Mol Sci.2021, 22(11):5503.
Int J Mol Sci.2023, 24(18):13713.
Pharmaceutics.2022, 14(3):564.
J Appl Toxicol.2020, 40(7):965-978.
Pharmaceuticals.2022, 15(4), 402.
Evid Based Complement Alternat Med.2021, 8707280.
Related and Featured Products
Planta Med. 1997 Feb;63(1):11-4.
In vitro antimutagenic effects of anthraquinone aglycones and naphthopyrone glycosides from Cassia tora.[Pubmed:
9063089 ]
The antimutagenic activity of a methanol extract of Cassia tora seeds against aflatoxin B1(AFB1) was demonstrated with the Salmonella typhimurium assay.
METHODS AND RESULTS:
The numbers of revertants per plate decreased significantly when this extract was added to the assay system using Salmonella typhimurium TA100 and/or TA98. The MeOH extract was then sequentially partitioned with CH2Cl2, n-BuOH and H2O. The CH2Cl2 and n-BuOH fractions possessed antimutagenic activity but the H2O fraction was inactive. Neither the MeOH extract nor its fractions were capable of inhibiting the direct-acting mutagen N-methyl-N'-nitro-N-nitrosoguanidine suggesting that these fractions may prevent the metabolic activation of AFB1 or scavenge the electrophilic intermediate capable of inducing mutations.
CONCLUSIONS:
Column chromatography using silica gel yielded pure chrysophanol, chryso-obtusin, and aurantio-obtusin from the CH2Cl2 fraction and Cassiaside and rubro-fusarin gentiobioside from the n-BuOH fraction. Each of these compounds demonstrated significant antimutagenic activity.
Arch Pharm Res. 1994 Dec;17(6):462-6.
Alaternin, cassiaside and rubrofusarin gentiobioside, radical scavenging principles from the seeds of Cassia tora on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical.[Pubmed:
10319159]
Radical scavenging principles on 1,1-diphenyl-2-picrylhydrazyl(DPPH) radical were isolated from the seeds of Cassia tora L.
METHODS AND RESULTS:
Assignments of the 1H- and 13C-NMR data showed the active components to be an anthraquinone, alaternin and two naphthopyrone glycosides, nor-rubrofusarin-6-beta-D-glucoside(Cassiaside) and rubrofusarin-6- -D-gentiobioside. Alaternin showed more potent radical scavenging effect than the others.
Planta Med. 1989 Jun;55(3):276-80.
New antihepatotoxic naphtho-pyrone glycosides from the seeds of Cassia tora.[Pubmed:
2740460 ]
METHODS AND RESULTS:
Two new naphtho-pyrone glycosides, 9-[(beta-D-glucopyranosyl-(1----6)-O-beta-D-glucopyranosyl)oxy]-10- hydroxy-7-methoxy-3-methyl-1H-naphtho[2,3-c]pyran-1 -one (5) and 6-[(alpha-apiofuranosyl-(1----6)-O-beta-D-glucopyranosyl)oxy]- rubrofusarin (6), together with Cassiaside (3) and rubrofusarin-6-beta-gentiobioside (4) were isolated from the seeds of Cassia tora L. Their structures were elucidated on the basis of chemical and spectral data.
CONCLUSIONS:
The naphtho-gamma-pyrone glycosides (3, 4, and 6) were found to have significant hepato-protective effects against galactosamine damage, which were higher than that of silybin from Silybum marianum.
J Ethnopharmacol. 2016 Sep 15;191:152-60.
Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against β-secretase and cholinesterases.[Pubmed:
27321278 ]
The basic goal of this study was to evaluate the anti-AD activities of C. obtusifolia and its major constituents. Previously, the extract of C. obtusifolia seeds, was reported to have memory enhancing properties and anti-AD activity to ameliorate amyloid β-induced synaptic dysfunction. However, the responsible components of C. obtusifolia seeds in an AD are currently still unknown.
METHODS AND RESULTS:
In this study, we investigated the inhibitory effects of C. obtusifolia and its constituents against acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and β-site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) enzyme activity. MATERIALS AND METHODS: In vitro cholinesterase enzyme assays by using AChE, BChE, and BACE1 were performed. We also scrutinized the potentials of Cassiae semen active component as BACE1 inhibitors via enzyme kinetics and molecular docking simulation. RESULTS: In vitro enzyme assays demonstrated that C. obtusifolia and its major constituents have promising inhibitory potential against AChE, BChE, and BACE1. All Cassiae semen constituents exhibited potent inhibitory activities against AChE and BACE1 with IC50 values of 6.29-109μg/mL and 0.94-190μg/mL, whereas alaternin, questin, and toralactone gentiobioside exhibited significant inhibitory activities against BChE with IC50 values of 113.10-137.74μg/mL.
CONCLUSIONS:
Kinetic study revealed that alaternin noncompetitively inhibited, whereas Cassiaside and emodin showed mixed-type inhibition against BACE1. Furthermore, molecular docking simulation results demonstrated that hydroxyl group of alaternin and emodin tightly interacted with the active site residues of BACE1 and their relevant binding energies (-6.62 and -6.89kcal/mol), indicating a higher affinity and tighter binding capacity of these compounds for the active site of BACE1.
3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No: CFN95021
CAS No: 1178-24-1
Price: $268/10mg
Lucidumol A
Catalog No: CFN95059
CAS No: 217476-73-8
Price: $318/5mg
Parvisoflavanone
Catalog No: CFN95077
CAS No: 49776-79-6
Price: $413/5mg
Licoricesaponin H2
Catalog No: CFN95173
CAS No: 118441-85-3
Price: $368/20mg
Nortrachelogenin-8'-O-beta-glucoside
Catalog No: CFN95234
CAS No: 858127-38-5
Price: $368/10mg
Isospinosin
Catalog No: CFN95350
CAS No: 89701-83-7
Price: $318/5mg
Naringin 6''-acetate
Catalog No: CFN95418
CAS No: 139934-60-4
Price: $318/5mg
Isorhamnetin-3-O-rutinoside-7-O-glucoside
Catalog No: CFN95494
CAS No: 55481-91-9
Price: $318/5mg
Benzylpropyl acetate
Catalog No: CFN95495
CAS No: 7492-40-2
Price: $318/5mg
12beta-Acetoxy-7beta-hydroxy-3,11,15,23-tetraoxo-5alpha-lanosta-8,20-dien-26-oic acid
Catalog No: CFN95515
CAS No: 1245946-62-6
Price: $318/5mg