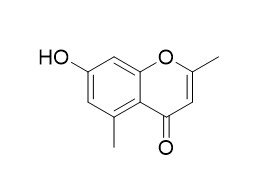
Altechromone A
Altechromone A has antimicrobial activity, it is active against Bacillus subtilis, Escherichia coli, Pseudomonas fluorescens and Candida albicans with the MICs of 3.9, 3.9, 1.8, and 3.9 ug/ml, respectively.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Ethnopharmacol.2017, 206:327-336
Int. J. Mol. Sci. 2022, 23(3),1696.
Int J Biol Macromol.2019, 126:653-661
Bulletin of Health Research2016, 44(4):279-286
Antioxidants.2022, 11(4), 67.
J Ethnopharmacol.2023, 309:116302.
Pathol Res Pract.2024, :260:155445.
Research on Crops.2017, 18(2)
Plant J.2021, 107(6):1711-1723.
Int J Mol Sci.2021, 22(12):6466.
Related and Featured Products
J Nat Prod. 2007 Jan;70(1):114-7.
Cytotoxic benzo[j]fluoranthene metabolites from Hypoxylon truncatum IFB-18, an endophyte of Artemisia annua.[Pubmed:
17253861 ]
METHODS AND RESULTS:
Two new benzo[j]fluoranthene-based secondary metabolites named daldinone C (1) and daldinone D (2), along with two known metabolites, Altechromone A and (4S)-5,8-dihydroxy-4-methoxy-alpha-tetralone, were isolated from the CHCl3/MeOH (1:1) extract of a solid culture of the endophyte Hypoxylon truncatum IFB-18 harbored inside the symptomless stem tissue of Artemisia annua. The structures of the new compounds were elucidated by MS and 1D and 2D NMR spectra and by X-ray diffraction analysis. Their absolute configurations were determined unambiguously by a combination of their CD data and the established exciton chirality rule.
CONCLUSIONS:
Compounds 1 and 2 were substantially cytotoxic against SW1116 cells, with IC50 values of 49.5 and 41.0 microM, respectively, comparable to that (37.0 microM) of 5-fluorouracil. The biosynthetic pathway for 1 and 2 was postulated with the natural occurrence of benzo[j]fluoranthene analogues discussed in brief.
World Journal of Microbiology & Biotechnology, 2009, 25(9):1677-1683.
Bioactive metabolites from Alternaria brassicicola ML-P08, an endophytic fungus residing in Malus halliana[Reference:
WebLink]
METHODS AND RESULTS:
A total of 48 strains were isolated from the normal tissues of Malus halliana and the EtOAc extracts of their cultures were subjected to primary antimicrobial screening against four test bacteria and three fungi. As a result, 22 strains exhibited antimicrobial activity against at least one test microbe. Among them, Alternaria brassicicola ML-P08 showing strong activity (MICs: 0.31–2.50 mg/ml) was selected for further investigation on its secondary metabolites. Bioassay-guided fractionation of the EtOAc extract of its liquid culture afforded seven compounds, which were identified as alternariol (1), alternariol 9-methyl ether (2), Altechromone A (3), herbarin A (4), cerevisterol (5), 3β,5α-dihydroxy-(22E,24R)-ergosta-7,22-dien-6-one (6) and 3β-hydroxy-(22E,24R)-ergosta-5,8,22-trien-7-one (7), respectively, by spectral means (MS, IR, 1H- and 13C-NMR).
CONCLUSIONS:
In vitro antimicrobial assay showed that compound 3 was substantially active against Bacillus subtilis, Escherichia coli, Pseudomonas fluorescens and Candida albicans with the MICs of 3.9, 3.9, 1.8, and 3.9 μg/ml, respectively. Compound 4 also showed pronounced antifungal activity against Trichophyton rubrum and C. albicans with MICs of both 15.6 μg/ml. In addition, compound 1 exhibited strong xanthine oxidase inhibitory activity with the IC50 of 15.5 μM, comparable to that of positive control, allopurinol (IC50: 10.7 μM).