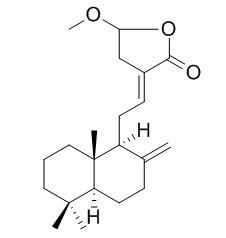
Coronarin D methyl ether
Coronarin D methyl ether has inhibitory effects on the increase in vascular permeability, nitric oxide production, and inducible nitric oxide synthase induction.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Science and Biotechnology2022, 10.1007.
ACS Pharmacol Transl Sci.2024, 7(2):395-405.
Regen Biomater.2023, 10:rbad077.
J Microbiol Biotechnol.2020, 30(2):178-186.
The Japan Society for Analytical Chemistry2017, 613-617
Elife.2021, 10:e68058.
Thorac Cancer.2023, 14(21):2007-2017.
Phytomedicine.2022, 102:154183.
Molecules.2019, 24(21):E3834
J Ethnopharmacol.2020, 249:112396
Related and Featured Products
Bioorg Med Chem Lett. 2011 Dec 15;21(24):7460-5.
Chemical constituents of the rhizomes of Hedychium coronarium and their inhibitory effect on the pro-inflammatory cytokines production LPS-stimulated in bone marrow-derived dendritic cells.[Pubmed:
22071304]
The rhizomes of Hedychium coronarium have been used for the treatment of inflammation, skin diseases, headache, and sharp pain due to rheumatism in traditional medicine.
METHODS AND RESULTS:
From this plant, three new labdane-type diterpenes 1-3, named coronarins G-I as well as seven known 4-10, coronarin D, Coronarin D methyl ether, hedyforrestin C, (E)-nerolidol, β-sitosterol, daucosterol, and stigmasterol were isolated. Their chemical structures were elucidated by mass, 1D- and 2D-nuclear magnetic resonance spectroscopy. They were evaluated for inhibitory effects on lipopolysaccharide-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. Among of them, compounds 1, 2, and 6 were significant inhibitors of LPS-stimulated TNF-α, IL-6, and IL-12 p40 productions with IC(50) ranging from 0.19±0.11 to 10.38±2.34 μM. The remains of compounds showed inactivity or due to cytotoxicity.
CONCLUSIONS:
These results warrant further studies concerning the potential anti-inflammatory benefits of labdane-type diterpenes from H. coronarium.
Nat Prod Res. 2008;22(14):1249-56.
Labdane diterpenes from the rhizomes of Hedychium coronarium.[Pubmed:
18932088]
METHODS AND RESULTS:
A new labdane diterpenoid, (E)-labda-8(17),12-dien-15,16-olide (1) together with eight known compounds, coronarin D (2), Coronarin D methyl ether (3), coronarin D ethyl ether (4), isocoronarin D (5), coronarin B (6), labda-8(17),11,13-trien-15,16-olide (7), (E)-labda-8(17),12-diene-15,16-dial (8) and 16-hydroxylabda-8(17),11,13-trien-15,16-olide (9), are isolated from the rhizomes of Hedychium coronarium. Compounds 2-4, 5 and 9 are isolated as mixtures of C-15, C-14 and C-16 epimers, respectively. Their structures are determined on the basis of their spectroscopic data.
CONCLUSIONS:
The epimeric mixtures of 2 and 3 have not been reported before. Some of them were evaluated for their cytotoxicity.