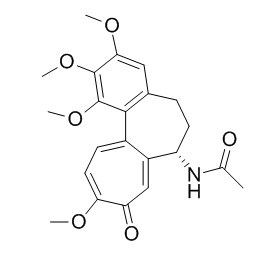
Colchicine
Colchicine is a tubulin inhibitor, and a microtubule polymerization inhibitor with an IC50 of 3 nM. It prevents amyloidosis in our high-risk population and that it can prevent additional deterioration of renal function in patients with amyloidosis who have proteinuria but not the nephrotic syndrome. Colchicine has anti-mitotic activity, it can be used to treat familial mediterranean fever, and as a lead compound for the generation of potent anti-cancer drugs.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Drug Chem Toxicol.2020, 1-12.
Phytomedicine.2019, 56:48-56
Evid Based Complement Alternat Med.2022, 2022:1307173.
Eur J Pharmacol.2023, 950:175772.
Industrial Crops and Products2018, 353-362
Biol Pharm Bull.2018, 41(11):1645-1651
Institute of Food Science & Technology2021, 18 December.
Onco Targets Ther.2017, 10:3467-3474
Molecules2022, 27(9):2992.
Chemistry of Plant Raw Materials2022, 20220210569.
Related and Featured Products
Med Res Rev. 2008 Jan;28(1):155-83.
Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin.[Pubmed:
17464966 ]
In this review, an attempt has been made to throw light on the mechanism of action of Colchicine and its different analogs as anti-cancer agents. Colchicine interacts with tubulin and perturbs the assembly dynamics of microtubules. Though its use has been limited because of its toxicity, Colchicine can still be used as a lead compound for the generation of potent anti-cancer drugs. Colchicine binds to tubulin in a poorly reversible manner with high activation energy. The binding interaction is favored entropically. In contrast, binding of its simple analogs AC or DAAC is enthalpically favored and commences with comparatively low activation energy. Colchicine-tubulin interaction, which is normally pH dependent, has been found to be independent of pH in the presence of microtubule-associated proteins, salts or upon cleavage of carboxy termini of tubulin. Biphasic kinetics of Colchicines-tubulin interaction has been explained in light of the variation in the residues around the drug-binding site on beta-tubulin. Using the crystal structure of the tubulin-DAMAColchicine complex, a detailed discussion on the pharmacophore concept that explains the variation of affinity for different Colchicine site inhibitors (CSI) has been discussed.
N Engl J Med. 1986 Apr 17;314(16):1001-5.
Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever.[Pubmed:
3515182 ]
METHODS AND RESULTS:
To determine whether Colchicine prevents or ameliorates amyloidosis in patients with familial Mediterranean fever, we followed 1070 patients with the latter disease for 4 to 11 years after they were advised to take Colchicine to prevent febrile attacks. Overall, at the end of the study, the prevalence of nephropathy was one third of that in a study conducted before Colchicine was used to treat familial Mediterranean fever. Among 960 patients who initially had no evidence of amyloidosis, proteinuria appeared in 4 who adhered to the prophylactic schedule and in 16 of 54 who admitted non-compliance. Life-table analysis showed that the cumulative rate of proteinuria was 1.7 percent (90 percent confidence limits, 0.0 and 11.3 percent) after 11 years in the compliant patients and 48.9 percent (18.8 and 79.0 percent) after 9 years in the noncompliant patients (P less than 0.0001). A total of 110 patients had overt nephropathy when they started to take Colchicine. Among 86 patients who had proteinuria but not the nephrotic syndrome, proteinuria resolved in 5 and stabilized in 68 (for more than eight years in 40). Renal function deteriorated in 13 of the patients with proteinuria and in all of the 24 patients with the nephrotic syndrome or uremia.
CONCLUSIONS:
We conclude that Colchicine prevented amyloidosis in our high-risk population and that it can prevent additional deterioration of renal function in patients with amyloidosis who have proteinuria but not the nephrotic syndrome.
New Engl. J. Med., 1974, 291(18): 934-7.
Colchicine Therapy for Familial Mediterranean Fever: A Double-Blind Trial[Reference:
WebLink]
METHODS AND RESULTS:
Eleven patients with long standing familial Mediterranean fever were studied in a double blind trial using daily Colchicine or placebo. During 60 courses of placebo, 38 attacks of familial Mediterranean fever occurred. In contrast, during 60 courses of Colchicine only seven attacks occurred (p < 0.001, by chi square test). The 7 attacks on Colchicine were evenly distributed throughout the study period. Although the initial dose was 3 tablets daily, most patients were maintained on 2 tablets of Colchicine per day. The efficacy of the drug in preventing attacks of familial Mediterranean fever was dose related. There were no major side effects from daily Colchicine except frequent loose stools, which were correctable by reduction of the dose.
CONCLUSIONS:
This study demonstrates that daily Colchicine is highly effective in preventing attacks in this disorder.
J Clin Invest. 1995 Aug;96(2):994-1002.
Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils.[Pubmed:
7543498 ]
METHODS AND RESULTS:
Since Colchicine-sensitive microtubules regulate the expression and topography of surface glycoproteins on a variety of cells, we sought evidence that Colchicine interferes with neutrophil-endothelial interactions by altering the number and/or distribution of selectins on endothelial cells and neutrophils. Extremely low, prophylactic, concentrations of Colchicine (IC50 = 3 nM) eliminated the E-selectin-mediated increment in endothelial adhesiveness for neutrophils in response to IL-1 (P < 0.001) or TNF alpha (P < 0.001) by changing the distribution, but not the number, of E-selectin molecules on the surface of the endothelial cells. Colchicine inhibited stimulated endothelial adhesiveness via its effects on microtubules since vinblastine, an agent which perturbs microtubule function by other mechanisms, diminished adhesiveness whereas the photoinactivated Colchicine derivative gamma-lumiColchicine was inactive. Colchicine had no effect on cell viability. At higher, therapeutic, concentrations Colchicine (IC50 = 300 nM, P < 0.001) also diminished the expression of L-selectin on the surface of neutrophils (but not lymphocytes) without affecting expression of the beta 2-integrin CD11b/CD18. In confirmation, L-selectin expression was strikingly reduced (relative to CD11b/CD18 expression) on neutrophils from two individuals who had ingested therapeutic doses of Colchicine.
CONCLUSIONS:
These results suggest that Colchicine may exert its prophylactic effects on cytokine-provoked inflammation by diminishing the qualitative expression of E-selectin on endothelium, and its therapeutic effects by diminishing the quantitative expression of L-selectin on neutrophils.
Nature, 2004, 428(6979):198-202.
Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain.[Pubmed:
15014504]
Microtubules are cytoskeletal polymers of tubulin involved in many cellular functions. Their dynamic instability is controlled by numerous compounds and proteins, including Colchicine and stathmin family proteins. The way in which microtubule instability is regulated at the molecular level has remained elusive, mainly because of the lack of appropriate structural data.
METHODS AND RESULTS:
Here, we present the structure, at 3.5 A resolution, of tubulin in complex with Colchicine and with the stathmin-like domain (SLD) of RB3. It shows the interaction of RB3-SLD with two tubulin heterodimers in a curved complex capped by the SLD amino-terminal domain, which prevents the incorporation of the complexed tubulin into microtubules. A comparison with the structure of tubulin in protofilaments shows changes in the subunits of tubulin as it switches from its straight conformation to a curved one.
CONCLUSIONS:
These changes correlate with the loss of lateral contacts and provide a rationale for the rapid microtubule depolymerization characteristic of dynamic instability. Moreover, the tubulin-Colchicine complex sheds light on the mechanism of Colchicine's activity: we show that Colchicine binds at a location where it prevents curved tubulin from adopting a straight structure, which inhibits assembly.