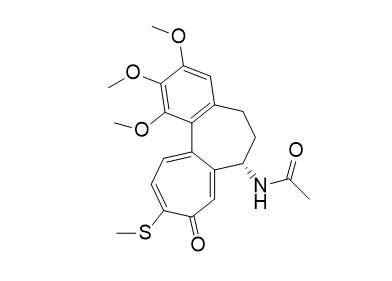
Thiocolchicine
Thiocolchicine is an effective inhibitor of tubulin polymerization with an IC50 of 2.5 μM and a Ki of 0.7 μM. Thiocolchicine induces cell apoptosis. Thiocolchicine can be used as an ADC cytotoxin in ADC technology.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Trop J Nat Prod Res2023, 7(12):5611-5615.
Acta Agriculturae Scandinavica2015, 381-383
Cells.2022, 11(8), 1311.
Int J Mol Sci.2022, 23(15):8687.
Int J Mol Sci.2018, 19(9):E2681
Enzyme Microb Technol.2022, 153:109941.
Antioxidants (Basel).2023, 12(12):2131.
Journal of Mushroom2023, 21(4):215-221.
Antioxidants (Basel).2021, 10(11): 1802.
J Biomol Struct Dyn.2022, 1-21.
Related and Featured Products
Anticancer Drug Des . 1998 Jan;13(1):19-33.
Antiproliferative activity of colchicine analogues on MDR-positive and MDR-negative human cancer cell lines[Pubmed:
9474240]
In this study the in vitro antitumor activity of a series of 20 colchicine analogues was tested and compared with colchicine and Thiocolchicine on three different human cancer cell lines, two of which express the multidrug-resistance (MDR) phenotype. At concentrations from 1 nM to 100 microM, all compounds tested inhibited cancer cell proliferation. The IC50 values indicate that the three fluorinated analogues were the most active compounds, with a similar decreasing order of potency (IDN 5005 > IDN 5079 > IDN 5080) on the two MDR-expressing cell lines, whereas Thiocolchicine was the most effective compound on the MDR-negative MDA-MB 231 cells. A strong correlation (r = 0.94; P = 0.004) was found between IC50 values obtained using the two MDR-positive cell lines. Conversely, IC50 values obtained in MDA-MB 231 cells did not show a significant correlation with MDR-positive cell lines, thereby suggesting some difference in the antiproliferative mechanism(s) of colchicine analogues. Cell cycle analysis of the most active analogues in breast cancer cells showed a relationship between cell cycle blocking activity and growth inhibition. The most active agents on the MDR-positive MCF7 ADRr cell line, after 24 h of culture, in terms of cell cycle blocking activity were the three fluorinated analogues. Interestingly, after 72 h, when the cell cycle block subsided, a consistent amount of DNA fragmentation was evident. The extent of cell cycle block, measured as the G2/G1 ratio, was significantly correlated with the apoptosis rate expressed as a percentage of DNA fragmentation on both cell lines, thereby suggesting that a large number of blocked cells underwent the apoptotic pathway.
Biochemistry . 1993 Jun 29;32(25):6470-6476.
The nitrogen of the acetamido group of colchicine modulates P-glycoprotein-mediated multidrug resistance[Pubmed:
8100149]
The substituents of drug molecules and the specific amino acid residues of P-glycoprotein (P-gp) implicated in drug/protein interactions are largely unknown. We have used a series of colchicine analogs modified on the A, B, and C rings to identify the discrete chemical groups on the colchicine molecule that are required for recognition by P-gp. For this, the toxicity of these analogs was tested on independent cell clones expressing either of the two mouse mdr genes, mdr1 and mdr3, known to confer multidrug resistance. Modifications of the methoxy groups on the A and C rings modulated cellular toxicity but had no effect on P-gp recognition; however, modifications at the C7 position of the B ring, in particular the removal of the nitrogen atom of the acetamido group, had a dramatic effect. Analogs bearing a hydrogen at that position were not substrates for P-gp. The importance of the nitrogen at C7 was independently verified in Thiocolchicine and allocolchicine analogs similarly modified, although overall levels of resistance to these compounds were somewhat reduced compared to their colchicine counterparts. The study of allocolchicine congeners bearing a six-carbon C ring and of two other analogs completely lacking a B ring suggested that intact B and C rings were important for interaction with P-gp. These results suggest that the structural determinants for cytotoxicity (tubulin binding) and P-gp recognition map to nonoverlapping sites in the colchicine analogs analyzed. Examination of calculated molar refractivities (CMR) revealed that only compounds showing CMR values greater than 9.7 were P-gp substrates.
Int Braz J Urol . 2016 Sep-Oct;42(5):1005-1009.
A prospective, randomized, single - blind study comparing intraplaque injection of thiocolchicine and verapamil in Peyronie's Disease: a pilot study[Pubmed:
24893912]
Objectives: To compare the response to tiocolchicine and verapamil injection in the plaque of patients with Peyronie's disease.
Materials and methods: Prospective, single-blind, randomized study, selecting patients who have presented Peyronie's disease for less than 18 months. Thiocolchicine 4mg or verapamil 5mg were given in 7 injections (once a week). Patients who had received any treatment for Peyronie's disease in the past three months were excluded. The parameters used were the International Index of Erectile Function (IIEF-5) score, analysis of the curvature on pharmaco-induced erections and size of the plaque by ultrasonography.
Results: Twenty-five patients were randomized, 13 received Thiocolchicine and 12 were treated with verapamil. Both groups were statistically similar. The mean curvature was 46.7o and 36.2o before and after Thiocolchicine, respectively (p=0.019) and 50.4o and 42.08o before and after verapamil, respectively (p=0.012). The curvature improved in 69% of patients treated with Thiocolchicine and in 66% of those who received verapamil. Regarding sexual function, there was an increase in the IIEF-5 from 16.69 to 20.85 (p=0.23) in the Thiocolchicine group. In the verapamil group the IIEF-5 score dropped from 17.50 to 16.25 (p=0.58). In the Thiocolchicine group, the plaque was reduced in 61% of patients. In the verapamil group, 8% presented decreased plaque size. No adverse event was associated to Thiocolchicine.
Conclusion: The use of Thiocolchicine in Peyronie's disease demonstrated improvement on penile curvature and reduction in plaque size. Thiocolchicine presented similar results to verapamil in curvature assessment. No significant side effects were observed with the use of tiocolchicine.
Anticancer Drug Des . 1998 Jan;13(1):19-33.
Antiproliferative activity of colchicine analogues on MDR-positive and MDR-negative human cancer cell lines[Pubmed:
9474240]
In this study the in vitro antitumor activity of a series of 20 colchicine analogues was tested and compared with colchicine and Thiocolchicine on three different human cancer cell lines, two of which express the multidrug-resistance (MDR) phenotype. At concentrations from 1 nM to 100 microM, all compounds tested inhibited cancer cell proliferation. The IC50 values indicate that the three fluorinated analogues were the most active compounds, with a similar decreasing order of potency (IDN 5005 > IDN 5079 > IDN 5080) on the two MDR-expressing cell lines, whereas Thiocolchicine was the most effective compound on the MDR-negative MDA-MB 231 cells. A strong correlation (r = 0.94; P = 0.004) was found between IC50 values obtained using the two MDR-positive cell lines. Conversely, IC50 values obtained in MDA-MB 231 cells did not show a significant correlation with MDR-positive cell lines, thereby suggesting some difference in the antiproliferative mechanism(s) of colchicine analogues. Cell cycle analysis of the most active analogues in breast cancer cells showed a relationship between cell cycle blocking activity and growth inhibition. The most active agents on the MDR-positive MCF7 ADRr cell line, after 24 h of culture, in terms of cell cycle blocking activity were the three fluorinated analogues. Interestingly, after 72 h, when the cell cycle block subsided, a consistent amount of DNA fragmentation was evident. The extent of cell cycle block, measured as the G2/G1 ratio, was significantly correlated with the apoptosis rate expressed as a percentage of DNA fragmentation on both cell lines, thereby suggesting that a large number of blocked cells underwent the apoptotic pathway.