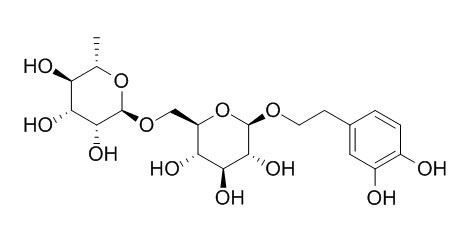
Forsythoside E
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2019, 24(2):E343
Asian J Beauty Cosmetol2021, 19(1): 57-64.
Planta Med.2023, 2192-2281
Mol Biol Rep.2024, 51(1):56.
Cell.2018, 172(1-2):249-261
Malaysian Journal of Analytical Sciences2022, 26(2):360-369.
Korean J. Medicinal Crop Sci.2018, 26(2):148-156
J of Dentistry & Oral Health2019, 2641-1962
Pharmaceutics.2021, 13(11):1839.
Revista Brasileira de Farmacognosia2024, 34:1091-1100.
Related and Featured Products
《Chinese Journal of Pharmaceutical Analysis》 2013-03
Determination of forsythoside E in Tanreqing injection by LC-MS/MS[Reference:
WebLink]
To establish a liquid chromatography-tandem mass spectrometric method(LC-MS/MS)for the determination of Forsythoside E in Tanreqing injection.
METHODS AND RESULTS:
After being diluted,the sample was separated on an Agilent SB-C18 column(2.1 mm×15 mm,3.5 μm)for gradient elution with a mobile phase of acetonitrile-0.01% formic acid water-solution at a flow rate of 0.3 mL·min-1.Then the processed sample was analyzed by tandem mass spectrometer with negative-electrospray ionization(ESI)source,monitored under a multiple reaction monitoring(MRM)mode.The calibration curve showed a good linearity in the range of 47.5-950 ng·mL-1.The average recoveries(n=3)of low,middle and high concentrations were 99.0%,103.0%,95.2% with RSDs of 3.4%,1.5% and 1.0%,respectively.The content of Forsythoside E in 13 batches of Tanreqing injection samples was in the range of 0.077-0.145 mg·mL-1.
CONCLUSIONS:
The developed method can be used for the quantitative determination of Forsythoside E in Tanreqing injection.