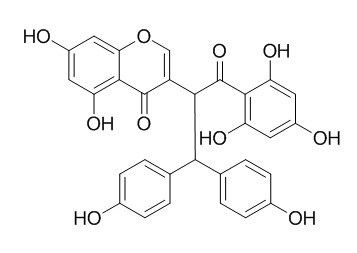
Chamaechromone
Chamaechromone has anti-HBV and insecticidal activity. The hydroxylation of Chamaechromone is inhibited by α-naphthoflavone, and predominantly catalyzed by recombinant human cytochrome P450 1A2.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Anat Rec (Hoboken).2021, 304(2):323-332.
Tumour Biol.2015, 36(9):7027-34
Microchemical Journal2022, 182: 107874.
Antioxidants2022, 11(2),234.
ACS Omega.2024, 9(12):14356-14367.
Saf Health Work.2019, 10(2):196-204
Front Chem.2022, 10:1048467.
Int J Med Sci.2020, 17(5):626-631
Biochem Biophys Res Commun.2018, 505(1):261-266
Int J Mol Sci.2019, 20(16):E4015
Related and Featured Products
J Ethnopharmacol. 2014;151(1):242-52.
Metabolites characterization of chamaechromone in vivo and in vitro by using ultra-performance liquid chromatography/Xevo G2 quadrupole time-of-flight tandem mass spectrometry.[Pubmed:
24189033]
Chamaechromone was a major component in the dried roots of Stellera chamaejasme with anti-HBV and insecticidal activity. Analysis of metabolic profile in vivo and in vitro plays a pivotal role to unravel how TCM works. And the metabolites of Chamaechromone might influence the effects and toxicity of Stellera chamaejasme. Moreover, the metabolic routes of Chamaechromone provide an important basis for toxicological safety evaluation. Until now, little is known about the metabolism of Chamaechromone. The current study was designed to characterize the whole metabolic pathways of Chamaechromone in vitro and in vivo.
METHODS AND RESULTS:
Twenty-four rats were randomly divided into four groups, including two oral administration groups (100mgkg(-1)), one intravenous injection group (5 mgkg(-1)), and one control group. The metabolites in rat urine and feces and bile were identified by UPLC/Q-TOF MS analysis and β-glucuronidase hydrolysis. Moreover, the possible metabolic mechanism was further confirmed by Phase I and Phase II metabolism and catechol-O-methyltransferase methylation in rat liver S9 fraction and degradation in rat intestinal bacteria. A total of 24 metabolites from Chamaechromone were detected and identified in vivo and in vitro, 20 of which were novel. And the major metabolic processes were hydroxylation, methylation, glucuronation, acetylation, dehydroxylation and degradation.
CONCLUSIONS:
The present study revealed the whole metabolic pathways of Chamaechromone in rat through both in vitro and in vivo experiments for the first time. And Chamaechromone could undergo extensive phase I and phase II metabolism in rat. These findings would provide an important basis for the further study and clinical application of Chamaechromone. In addition, the results of this work have showed the feasibility of the UPLC/Q-TOF-MS approach for rapid and reliable characterization of metabolites.
Planta Med. 2014 Apr;80(6):493-7.
Metabolism of chamaechromone in vitro with human liver microsomes and recombinant human drug-metabolizing enzymes.[Pubmed:
24687737]
Chamaechromone is a major component in the dried roots of Stellera chamaejasme with antihepatitis B virus and insecticidal activity.
METHODS AND RESULTS:
In this study, metabolic profiles of Chamaechromone were investigated in human liver microsomes. One monohydroxide and two monoglucuronides of Chamaechromone were identified. The enzyme kinetics for both hydroxylation and glucuronidation were fitted to the Michaelis-Menten equation. The hydroxylation of Chamaechromone was inhibited by α-naphthoflavone, and predominantly catalyzed by recombinant human cytochrome P450 1A2, whereas the glucuronidation was inhibited by quercetin, 1-naphthol, and fluconazole, and mainly catalyzed by recombinant human UDP-glucuronosyltransferase 1A3, 1A7, 1A9, and 2B7.