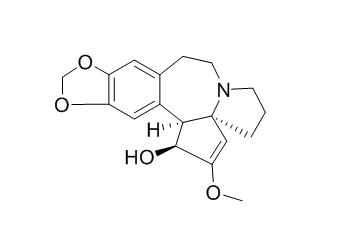
Cephalotaxine
Cephalotaxine is an antiviral as well as antitumor agent. It is useless for the treatment of infection by flaviviruses, but potentially useful in combined therapy against hepatitis B.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Antioxidants (Basel).2022, 11(12):2327.
J Agric Food Chem.2015, 63(44):9869-78
Nat Prod Sci.2018, 24(3):206
Life Sci.2018, 209:498-506
J of Advanced Scientific R.2020, 11(3), p109-120.
Int J Cosmet Sci.2019, 41(1):12-20
VNU Journal of Science2023, No. 20.
Food Chem.2023, 427:136647.
Horticulture Research2020, 7:111.
Int. J. Mol. Sci.2022, 23(8), 4130.
Related and Featured Products
Planta Med. 2007 Jun;73(6):552-8.
Effect of cantharidin, cephalotaxine and homoharringtonine on [Pubmed:
17458779 ]
The effect as antiviral agents versus viral hepatitis B and C of three compounds purified from natural products commonly used as remedies in traditional Chinese medicine, cantharidin, Cephalotaxine and homoharingtonine, was investigated.
METHODS AND RESULTS:
To assess the activity of these compounds against flavivirus, we used bovine viral diarrhoea virus (BVDV) as a surrogate for hepatitis C virus (HCV). Anti-BVDV activity was determined by reduction in BVDV-RNA production and protection of infected embryonic bovine trachea (EBTr) cells against the cytopathic effect of BVDV. The effect versus hepatitis B virus (HBV) was investigated by measuring HBsAg and HBV-DNA release from hepatoblastoma HepG2 2.2.15 cells infected with HBV. As positive control we used the standard anti-HBV and anti-HCV drugs, lamivudine and ribavirin, respectively. Up to 100 microM lamivudine and ribavirin did not induce cell toxicity, whereas they induced dose-dependent anti-HBV and anti-BVDV effects, respectively. In the same range, cantharidin, Cephalotaxine and homoharringtonine induced toxicity in EBTr cells and had no protective effect against BVDV.
CONCLUSIONS:
In contrast, they were able to inhibit HBV production at concentrations 10- to 100-fold lower than those inducing cell toxicity, which suggests that they are useless for the treatment of infection by flaviviruses, but potentially useful in combined therapy against hepatitis B.
Nat Prod Commun. 2016 Jan;11(1):57-62.
Molecular Docking and Binding Mode Analysis of Plant Alkaloids as in vitro and in silico Inhibitors of Trypanothione Reductase from Trypanosoma cruzi.[Pubmed:
26996020]
Trypanothione reductase (TryR) is a key enzyme in the metabolism of Trypanosoma cruzi, the parasite responsible for Chagas disease. The available repertoire of TryR inhibitors relies heavily on synthetic substrates of limited structural diversity, and less on plant-derived natural products.
METHODS AND RESULTS:
In this study, a molecular docking procedure using a Lamarckian Genetic Algorithm was implemented to examine the protein-ligand binding interactions of strong in vitro inhibitors for which no X-ray data is available. In addition, a small, skeletally diverse, set of natural alkaloids was assessed computationally against T. cruzi TryR in search of new scaffolds for lead development. The preferential binding mode (low number of clusters, high cluster population), together with the deduced binding interactions were used to discriminate among the virtual inhibitors.
CONCLUSIONS:
This study confirms the prior in vitro data and proposes quebrachamine, Cephalotaxine, cryptolepine, (22S,25S)-tomatidine, (22R,25S)-solanidine, and (22R,25R)-solasodine as new alkaloid scaffold leads in the search for more potent and selective TryR inhibitors.