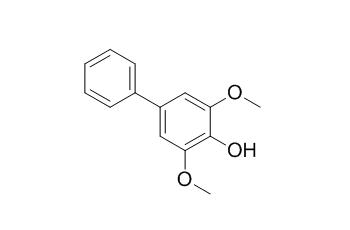
Aucuparin
Aucuparin is a phytoalexin, it shows significant scavenging activity by the ABTS and FRAP assays. Aucuparin exhibits potent inhibitory activity against fMLP-induced superoxide (O(*-)(2)) production by human neutrophils with the IC(50) value of 17.0+/-6.8 microM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int Immunopharmacol.2024, 141:112906.
Korean J. of Food Sci. and Tech2016, 172-177
Food Bioscience2023, 56:103311.
Neuropharmacology2019, 151437
Int J Mol Sci.2018, 19(9):E2601
Redox Rep.2024, 29(1):2392329.
Korean J of Crop Science2019, 452-458
Environ Toxicol.2019, 34(12):1354-1362
Journal of Chromatography A2020, 460942
Mediators Inflamm.2016, 2016:7216912
Related and Featured Products
Planta. 2012 Jan;235(1):217-23.
Endogenous hydrogen peroxide is a key factor in the yeast extract-induced activation of biphenyl biosynthesis in cell cultures of Sorbus aucuparia.[Pubmed:
22086110]
Biphenyls are unique phytoalexins produced by plants belonging to Pyrinae, a subtribe of the economically important Rosaceae family. The formation of Aucuparin, a well-known biphenyl, is induced by yeast extract (YE) in cell cultures of Sorbus aucuparia. However, the molecular mechanism underlying YE-induced activation of biphenyl biosynthesis remains unknown.
METHODS AND RESULTS:
Here we demonstrate that the addition of YE to the cell cultures results in a burst of reactive oxygen species (ROS; H(2)O(2) and O(2) (-)), followed by transcriptional activation of the biphenyl synthase 1 gene (BIS1) encoding the key enzyme of the biphenyl biosynthetic pathway and Aucuparin accumulation. Pretreatment of the cell cultures with ROS scavenger dihydrolipoic acid and NADPH oxidase-specific inhibitor diphenylene iodonium abolished all of the above YE-induced biological events. However, when the cell cultures was pretreated with superoxide dismutase specific inhibitor N,N-diethyldithiocarbamic acid, although O(2) (-) continued to be generated, the H(2)O(2) accumulation, BIS1 expression and Aucuparin production were blocked. Interestingly, exogenous supply of H(2)O(2) in the range of 0.05-10 mM failed to induce Aucuparin accumulation.
CONCLUSIONS:
These results indicate that endogenous generation of H(2)O(2) rather than that of O(2) (-) is a key factor in YE-induced accumulation of biphenyl phytoalexins in cell cultures of S. aucuparia.
Chem Biodivers. 2009 May;6(5):774-8.
A new dibenzofuran and further constituents from the stems of Pourthiaea lucida with inhibitory activity on superoxide generation by neutrophils.[Pubmed:
19479843 ]
METHODS AND RESULTS:
A new dibenzofuran, lucidafuran (1), was isolated from the stems of Pourthiaea lucida, together with eight known compounds. The structure of this new compound was determined through NMR and mass-spectrometric analyses.
CONCLUSIONS:
Among the isolated compounds, lucidafuran (1) and Aucuparin (3) exhibited potent inhibitory activity against fMLP-induced superoxide (O(*-)(2)) production by human neutrophils with IC(50) values of 18.7+/-4.4 and 17.0+/-6.8 microM, resp.
Zhongguo Zhong Yao Za Zhi. 2008 Aug;33(16):1982-5.
Antioxidant xanthones from Securidaca inappendiculata.[Pubmed:
19086633]
To study the antioxidant constituents from the roots of Securidaca inappendiculata.
METHODS AND RESULTS:
The bioassay-guided isolation of antioxidant constituents was carried out by the column chromatographic techniques. The combination of IR, MS, NMR and 2D-NMR spectroscopics methods was used to identify their structures. Two new xanthones, 1, 2, 5-trihydroxy-6, 8-dimethoxy-9H-xanthen-9-one(1), 1, 5-dihydroxy-2, 6, 8-trimethoxy-9H-xanthen-9-one (2), along with seven known ones, 3, 8-dihydroxy-1, 4-dimethoxy-9H-xanthen-9-one(3), 4, 6-dihydroxy-1, 5, 7-trimethoxy-9H-xanthen-9-one(4), 7-hydroxy-1, 2, 3, 8-tetramethoxy-9H-xanthen- 9-one(5), 1, 7-dihydroxy-9H-xanthen-9-one(6), 4-hydroxy-3, 7-dimethoxy-9H-xanthen-9-one(7), 1,7-dimethoxy-9H-xanthen-9-one(8) and Aucuparin(9), were isolated from the roots of S. inappendiculata.
CONCLUSIONS:
Compounds 1 and 2 were new xanthones, and compound 3 was isolated as a natural product for the first time, and compounds 4 and 6 were isolated for the first time from this genus. The antioxidant activities of all compounds were evaluated by ABTS, FRAP and DPPH assays respectively. Compound 9 showed significant activity by the ABTS and FRAP assays. Compound 1 showed significant activity with IC50 value of 0.31 mg x L(-1) in DPPH assay. Scavenging capacity of all compounds determined by all assays were well correlated between ABTS and FRAP assay (r = 0.9555).