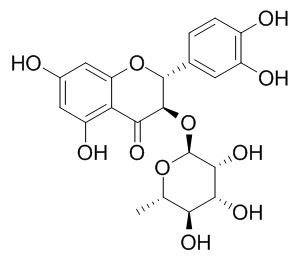
Astilbin
Astilbin has insecticidal, antioxidant, antibacterial, and anti-inflammatory activities, it may act as an efficient therapeutic agent for arthritis like cyclosporine A but with less toxicity, its mechanism includes a selective suppression on lymphocyte functions via reducing MMP and NO production. Astilbin can exert an early renal protective role to diabetic nephropathy (DN), inhibit production of transforming growth factor-beta1 (TGF-beta1) and connective tissue growth factor (CTGF).Astilbin also alleviates contact hypersensitivity through a unique mechanism involving a negative cytokine regulation through stimulating IL-10, which is distinct from the immunosuppressant cyclosporin A.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J of Advanced Scientific R.2020, 11(3), p109-120.
Phytomedicine.2021, 93:153789.
Trop J Nat Prod Res.2019, 3(1):6-9
Chem Biol Interact.2024, 394:110995.
J Cell Mol Med.2023, 27(10):1423-1435.
Front Pharmacol.2022, 13:919230.
Bulletin of Health Research2016, 44(4):279-286
Industrial Crops and Products2024, 129:119014
Invest New Drugs.2017, 35(2):166-179
J Appl Biol Chem.2021, 64(3),263?268
Related and Featured Products
J Ethnopharmacol. 2006 Jun 30;106(2):272-8.
Isolation and in vitro antibacterial activity of astilbin, the bioactive flavanone from the leaves of Harungana madagascariensis Lam. ex Poir. (Hypericaceae).[Pubmed:
16483735 ]
Harungana madagascariensis is well known for its topical antibacterial properties used in the elaboration of a lot of skin hygiene products. The aim of this study was, on the one hand, to evaluate the in vitro antibacterial activities of aqueous, ethanolic and ethyl acetate crude extracts of Harungana madagascariensis leaves against bacterial strains representative of skin microflora and, on the other hand, to determine the chemical structure of the active compound. Only the ethyl acetate leaf extract presented important antibacterial activity.
METHODS AND RESULTS:
Its fractionation was carried out by column chromatography using silica gel 60 and it yielded 11 fractions. A bioautographic method, revealed in these fractions the presence of a flavanone as the active compound Astilbin or 3-O-alpha-L-rhamnoside-5,7,3',4'-tetrahydroxydihydroflavonol which was identified on the basis of its spectroscopic data. Concerning the antibacterial activity against the representative skin microflora of the armpit and feet, MIC and MBC ranged from 25 to 250 and 100 to 750 microg ml-1, respectively.
CONCLUSIONS:
The results showed that some bacteria considered to be responsible for bad odours at the armpit and feet levels, were destroyed at 200 microg ml-1 (MBC), a concentration sparing most of the useful saprophytic microflora. The minimal inhibitory quantity (MIQs) of Astilbin ranged from 50 to 100 microg.
Food Chem.,2009,115(1):297-303.
Antioxidant activity of Rhizoma Smilacis Glabrae extracts and its key constituent-astilbin.[Reference:
WebLink]
Rhizoma Smilacis Glabrae is widely consumed by Chinese as functional food and in folk medicine for its medicinal properties. In this study, methanol and water extracts of Rhizoma Smilacis Glabrae were prepared. The water extract was further divided into polysaccharide and supernatant fractions.
METHODS AND RESULTS:
Constituents in different extracts were analysed by capillary electrophoresis, and levels of total phenolics were also determined using the Folin-Ciocalteu method. Astilbin, the main constituent in the herb, was isolated and purified. Different antioxidant tests were employed to evaluate the antioxidant activities of the extracts and the isolated Astilbin, and the results were compared with two commonly used synthetic antioxidants-butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). Methanol, water extract and supernatant fraction showed concentration dependent antioxidant activity while polysaccharide didn’t show any antioxidant activity.
CONCLUSIONS:
Purified Astilbin showed the strongest antioxidant activity in comparison to any other extracts.
Pest Manag Sci. 2002 May;58(5):503-7.
Biological activity of astilbin from Dimorphandra mollis against Anticarsia gemmatalis and Spodoptera frugiperda.[Pubmed:
11997979 ]
METHODS AND RESULTS:
Astilbin was isolated in high yield from Dimorphandra mollis, and its insecticidal and growth inhibiting activity by stomach ingestion were evaluated against Anticarsia gemmatalis and Spodoptera frugiperda. The insecticidal activity of Astilbin, the weight reduction of the larval phase and the prolongation of the larval and pupal phases were verified for both species.
CONCLUSIONS:
Astilbin was identified on the base of its NMR, MS and physical data.
Food Chem Toxicol. 2014 Jan;63:104-10.
Astilbin protects diabetic rat heart against ischemia-reperfusion injury via blockade of HMGB1-dependent NF-κB signaling pathway.[Pubmed:
24211745]
Astilbin, a flavonoid compound was isolated from the rhizome of Smilax china L.
METHODS AND RESULTS:
In this study, we investigated the anti-myocardial ischemia and reperfusion (I/R) injury effect of Astilbin on diabetic rats in vivo and elucidated the potential mechanism in vitro. The results showed that Astilbin significantly attenuated hypoxia-induced cell injury in a concentration-dependent manner. Treatment of H9c2 cells with Astilbin at 15 μM blocked nuclear factor kappaB (NF-κB) phosphorylation by blocking High-mobility group box protein 1 (HMGB1) expression. Treatment of diabetic rats with Astilbin by intravenous injection (i.v.) at a single dose of 50 mg/kg protected the rats from myocardial I/R injury as indicated by decreasing infarct volume, improving hemodynamics and reducing myocardial damage, and also lowered serum levels of pro-inflammatory factors, reduced HMGB1 and phosphorylated NF-κB expression in ischemic myocardial tissue from diabetic rats. Additionally, treatment of diabetic rats with Astilbin at dose of 50 mg/kg by i.v. for continuous 14 days attenuated cardiac remodeling in the model myocardial I/R injury.
CONCLUSIONS:
These protective effects suggested that Astilbin might be due to block of the myocardial inflammatory cascade via the HMGB1-dependent NF-κB signaling pathway.
J Pharm Pharmacol. 2004 Apr;56(4):495-502.
Astilbin prevents concanavalin A-induced liver injury by reducing TNF-alpha production and T lymphocytes adhesion.[Pubmed:
15104095]
The aim of this study was to evaluate the effect of Astilbin on concanavalin A (Con A)-induced hepatitis, a T cell-dependent model of liver injury.
METHODS AND RESULTS:
Con A administration resulted in a severe liver injury in mice, with a strong increment in spleen cell adhesion and liver infiltration of T cells, as well as in tumour necrosis factor (TNF)-alpha production. Against this liver injury, Astilbin significantly inhibited the elevation in transaminase activity, reduced the TNF-alpha production, and improved the histological changes, including inflammatory infiltration, hepatocyte necrosis and degeneration and Kupffer cell hyperplasia. In addition, Astilbin inhibited the adhesion of spleen cells and purified T lymphocytes isolated from the liver-injured mice to fibronectin, laminin and type IV collagen.Moreover, the adhesion of human Jurkat T cells to endothelial cell line ECV-304 was also inhibited by Astilbin.
CONCLUSIONS:
These results suggest that the improvement of the T cell-mediated liver injury by Astilbinmay be related to the reduction in TNF-alpha production and in T cell adhesion to extracellular matrices and endothelial cells.
Food Chem Toxicol . 2018 Apr;114:227-236.
Astilbin ameliorates cisplatin-induced nephrotoxicity through reducing oxidative stress and inflammation[Pubmed:
29471006]
Abstract
Oxidative stress and inflammation are considered to be the main pathogenesis of cisplatin nephrotoxicity. Astilbin, a flavonoid with anti-oxidation and anti-inflammation function, has been used to treat heavy metal induced kidney injury. In this study, we investigated the protective effects of Astilbin on cisplatin-induced nephrotoxicity and its underlying mechanisms. Our results showed that Astilbin markedly inhibited cisplatin-induced cell apoptosis and recovered cell growth. Astilbin significantly decreased reactive oxygen species (ROS) accumulation and alleviated ROS-induced activation of p53, MAPKs and AKT signaling cascades, which in turn attenuated cisplatin-induced HEK-293 cell apoptosis. Astilbin effectively enhanced NRF2 activation and transcription of its targeting antioxidant genes to reduce ROS accumulation in cisplatin-induced HEK-293 cells. Furthermore, we found that Astilbin obviously suppressed tumor necrosis factor alpha (TNF-α) expression and NF-κB activation, and also inhibited the expression of induced nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Finally, we confirmed that the effect of Astilbin to improve renal oxidative stress and inflammation in cisplatin induced acute nephrotoxic mice. In conclusion, our study suggests that Astilbin could ameliorate the cisplatin-induced nephrotoxicity by reducing oxidative stress and inflammation.
Keywords: Astilbin; Cisplatin nephrotoxicity; Inflammation; NRF2; Oxidative stress.
Planta Med. 2009 Nov;75(14):1470-5.
Effect of astilbin on experimental diabetic nephropathy in vivo and in vitro.[Pubmed:
19644810 ]
Astilbin, a flavonoid compound, was isolated from the rhizome of Smilax glabra Roxb. This study was conducted to investigate the efficacy of Astilbin on experimental diabetic nephropathy (DN) in vivo and in vitro and its possible mechanisms.
METHODS AND RESULTS:
Astilbin was added in high glucose stimulated HK-2 cells, streptozotocin-induced experimental DN, randomized to receive intragastric ( I. G.) Astilbin to observe its anti-renal lesion effect. Results showed that Astilbin inhibited high glucose stimulated HK-2 cell production of transforming growth factor-beta1 (TGF-beta1) and connective tissue growth factor (CTGF) in vitro, especially CTGF; analogic results was also found in vivo. I. G. of Astilbin 2.5 mg/kg or 5 mg/kg significantly ameliorated renal function, reduced kidney index, while it increased body weight and survival time in animals. In addition there was no significant difference in blood glucose level between the STZ-treated group and the Astilbin groups. Furthermore, Astilbin ameliorated the pathological progress of renal morphology. Astilbin can exert an early renal protective role to DN, inhibit production of TGF-beta1 and especially of CTGF.
CONCLUSIONS:
We suggest that Astilbin inhibition of CTGF may be a potential target in DN therapy. This work provides the first evidence for Astilbin as a new candidate of DN therapeutic medicine.
J Allergy Clin Immunol. 2005 Dec;116(6):1350-6.
Astilbin inhibits contact hypersensitivity through negative cytokine regulation distinct from cyclosporin A.[Pubmed:
16337470]
IL-10 is known as a negative regulator for inflammatory diseases, including contact dermatitis. However, only a few drug candidates are reported to induce endogenous IL-10.
We sought to elucidate a new mechanism underlying the immunosuppressive properties of Astilbin through negative cytokine regulation in comparison with the effective pattern with cyclosporin A.
METHODS AND RESULTS:
Contact hypersensitivity was induced in mice with picryl chloride. Lymph node cells were isolated for adoptive transfer and cytokine assays.
Astilbin significantly inhibited contact hypersensitivity when given in the elicitation phase but not in the sensitization phase, whereas cyclosporin A inhibited both phases. Lymph node cells from donor mice administered Astilbin failed to adoptively transfer the hypersensitivity. Astilbin in vivo remarkably induced IL-10 expression in lymph node cells at an earlier time and decreased TNF-alpha and IFN-gamma expression at a later time. Furthermore, the in vivo neutralization of IL-10 significantly impaired the effect of Astilbin on contact hypersensitivity. In the isolated lymphocytes sensitized with picryl chloride in vivo and challenged with trinitrobenzene-sulfonic acid in vitro, Astilbin did not affect the cell proliferation but modulated the above cytokine profiles as its in vivo effect in a concentration-dependent manner and furthermore significantly enhanced the expressions of suppressor of cytokine signaling 1 and 3. On the other hand, cyclosporin A strongly inhibited proinflammatory cytokine production but influenced neither IL-10 nor downstream suppressor of cytokine signaling 1 and 3 expression.
CONCLUSIONS:
Astilbin alleviates contact hypersensitivity through a unique mechanism involving a negative cytokine regulation through stimulating IL-10, which is distinct from the immunosuppressant cyclosporin A.
Pharmacol Res. 2001 Aug;44(2):135-9.
Astilbin selectively facilitates the apoptosis of interleukin-2-dependent phytohemagglutinin-activated Jurkat cells.[Pubmed:
11516264 ]
The present study examined the relationship between the activation of T cells and the apoptosis-facilitating effect of Astilbin on them.
METHODS AND RESULTS:
By the stimulation of PHA, a remarkable IL-2 production was detected in the supernatant of Jurkat cells after 120 h among 72--144 h incubation. This kinetics was quite in accordance with that of Astilbin-induced apoptosis of Jurkat cells, where 1 h-exposure of the PHA-activated cells to Astilbin caused a significantly increased apoptosis in a dose-dependent manner. To the Jurkat cells that had been cultivated for 72--144 h without PHA, however, Astilbin did not show any facilitation of the cell apoptosis. Pre-treatment by cyclosporine A simultaneously with PHA dose-dependently lowered the IL-2 production and susceptibility of the cells to Astilbin, while the treatment after 120 h of PHA-activation did not. The exogenous IL-2 treatment after 72 h of PHA-activation significantly and dose-dependently raised the susceptibility of the Jurkat cells to Astilbin.
CONCLUSIONS:
These results indicated the dependency of the apoptosis-facilitating effect of Astilbin on appropriate status of activated T lymphocytes with a relation to IL-2 production. This characteristic of Astilbin may be of great significance for the treatment of a variety of immunologically related diseases.
Biochem Biophys Res Commun. 2014 Apr 4;446(2):529-34.
Induction of TGF-β and IL-10 production in dendritic cells using astilbin to inhibit dextran sulfate sodium-induced colitis.[Pubmed:
24613838]
Astilbin, a major bioactive compound from Rhizoma smilacis glabrae, has been reported to possess anti-inflammatory properties.
METHODS AND RESULTS:
Our study first evaluated Astilbin on dextran sulfate sodium (DSS)-induced acute colitis in mice. By intraperitoneal injection of Astilbin, the severity of colitis was attenuated, and the serum levels of IL-10 and TGF-β were increased. Using flow cytometry, a higher number of IL-10(+) dendritic cells (DCs) and TGF-β(+) DCs and a lower number of CD86(+) DCs, IL-12 p40(+) DCs, and IL-1β(+) DCs were detected in the spleen of mice with colitis after Astilbin treatment. The administration of Astilbin also resulted in the upregulation of CD103(+) expression in colonic DCs. In a coculture system, murine bone marrow-derived DCs pretreated with Astilbin resulted in an enhanced production of CD4(+)CD25(+)Foxp3(+) T cells.
CONCLUSIONS:
The results of this study show that Astilbin could be a candidate drug for inflammatory bowel disease by mediating the regulatory functions of DCs.
Inflamm Res. 2003 Aug;52(8):334-40.
Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes.[Pubmed:
14504671 ]
To examine the therapeutic effects of Astilbin, a flavanoid isolated from Rhizoma Smilacis Glabrae, on arthritis and to compare it with cyclosporine A (CsA).
METHODS AND RESULTS:
Type II collagen-induced arthritis in mice and its in vitro assays for proliferation, matrix metalloproteinase (MMP) and NO production were performed.
Astilbin dose-dependently inhibited the footpad swelling, arthritic incidence, and clinical scores without influencing the body weights, while CsA showed strong inhibition with a significant weight loss. Histological examination revealed marked inflammatory damage in arthritic mice including joint swelling, synovial hyperplasia, and cartilage destruction. Against these, an intact joint structure was maintained in Astilbin-treated or CsA-treated mice. In isolated spleen cells from arthritic mice, increased potentials in proliferation, NO production, and MMP-2 and 9 activities were suppressed dose-dependently by the oral administration of Astilbin. Additionally, Astilbin showed neither any cytotoxicity to nor influence on Con A-induced proliferation of spleen cells from naive mice, while CsA showed a dose-dependent cytotoxicity and inhibition of the proliferation.
CONCLUSIONS:
Astilbin may act as an efficient therapeutic agent for arthritis like CsA but with less toxicity. Its mechanism includes a selective suppression on lymphocyte functions via reducing MMP and NO production.