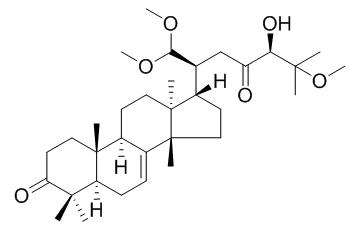
Aphagranin A
Aphagranin A exhibits strong antiproliferative activity against the growth of six lines of human cancer cells (MCF-7, A549, HepG2, Bel-7402, SGC-7901, and BGC-823.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Exp Biol Med (Maywood).2019, 244(18):1665-1679
Antioxidants (Basel).2022, 11(10):1929.
Microb Biotechnol.2021, 14(5):2009-2024.
Chemistry of Plant Raw Materials2019, 4:135-147
EXCLI J.2023, 22:482-498.
J Agric Food Chem.2016, 64(35):6783-90
Front Pharmacol.2021, 12:607403.
Org Biomol Chem.2017, 15(31):6483-6492
Korean J of Food Science&Technology 2017, 49(2):146-150
Gene.2022, 815:146178.
Related and Featured Products
Tetrahedron Letters, 2012, 53(14):1705-9.
A pair of tirucallane C27-triterpenoid cyclopentenone epimers from the stem barks of Aphanamixis grandifolia[Reference:
WebLink]
METHODS AND RESULTS:
Two novel tirucallane C27-triterpenoid epimers, aphagranins A (1) and B (2), featuring an unprecedented enolized cyclopentenone presented in the side-chain at C-17, were isolated from the stem barks of Aphanamixis grandifolia.
Extensive spectroscopic analyses helped the establishment of the structures of the two isolates, whose absolute configurations were determined using density functional theory (DFT) calculations of optical rotation, and electronic circular dichroism (ECD).
CONCLUSIONS:
Remarkable discrepancies in the inhibitory activities against the growth of six lines of human cancer cells (MCF-7, A549, HepG2, Bel-7402, SGC-7901, and BGC-823) were found for the two epimers: with IC50 less than 10 μM, Aphagranin A exhibited much stronger antiproliferative activity than aphagranin B, showing no such activities with IC50 over 20 μM.
Magn Reson Chem. 2011 Jul;49(7):450-7.
Complete 1H and 13C NMR data assignment of protolimonoids from the stem barks of Aphanamixis grandifolia.[Pubmed:
21590730 ]
METHODS AND RESULTS:
Seven new protolimonoids, named aphagranins A-G (1-7), along with four known compounds, were isolated from the ethanol extract of the stem barks of Aphanamixis grandifolia.
CONCLUSIONS:
Structure elucidation and signal assignments were achieved on the basis of spectral and chemical evidences.