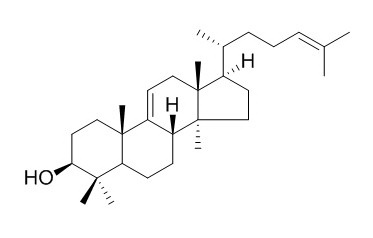
Parkeol
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Microbiol.2020, 11:583594.
Spectrochim Acta A2019, 210:372-380
Viruses2023, 15(6), 1377
Hum Exp Toxicol.2017, 36(11):1169-1176
Chem Biodivers.2023, 20(10):e202300741.
Front Immunol.2018, 9:2091
Agriculture2022, 12(2),227.
Molecules.2016, 21(6)
Biomol Ther (Seoul).2024, 32(2):214-223.
Korean Journal of Pharmacognosy2018, 49(1):76-83
Related and Featured Products
Org Lett. 2012 Oct 19;14(20):5222-5.
Protein engineering of Saccharomyces cerevisiae oxidosqualene-lanosterol cyclase into parkeol synthase.[Pubmed:
23043506]
METHODS AND RESULTS:
A Saccharomyces cerevisiae oxidosqualene-lanosterol cyclase mutant, ERG7(T384Y/Q450H/V454I), produced Parkeol but not lanosterol as the sole end product. Parkeol undergoes downstream metabolism to generate compounds 9 and 10. In vitro incubation of Parkeol produced a product profile similar to that of the in vivo experiment.
CONCLUSIONS:
In summary, Parkeol undergoes a metabolic pathway similar to that of cycloartenol in yeast but distinct from that of lanosterol in yeast, suggesting that two different metabolic pathways of postoxidosqualene cyclization may exist in S. cerevisiae.
J Oleo Sci. 2010;59(7):351-60.
Triterpene alcohol and fatty acid composition of shea nuts from seven African countries.[Pubmed:
20513968]
METHODS AND RESULTS:
The content and composition of triterpene alcohol fractions of the non-saponifiable lipids (NSL) along with the fatty acid composition of the kernel fats (n-hexane extracts) of the shea tree (Vitellaria paradoxa; Sapotaceae) were determined for 36 samples from seven sub-Saharan countries: Cote d' Ivoire, Ghana, Nigeria, Cameroun, Chad, Sudan, and Uganda. The fat content of the kernels, proportion of NSL in the fats, and triterpene alcohols in the NSL are in the range of 30-54, 2-12, and 22-72%, respectively.
CONCLUSIONS:
The triterpene alcohol fractions contained alpha-amyrin (1), beta-amyrin (2), lupeol (3), and butyrospermol (4) as the major constituents along with minor or trace amounts of psi-taraxasterol (5), taraxasterol (6), Parkeol (7), 24-methylene-24-dihydroParkeol (8), 24-methylenecycloartanol (9), dammaradienol (10), and 24-methylenedammarenol (11). Fatty acid composition is dominated by stearic (28-56%) and oleic (34-61%) acids.