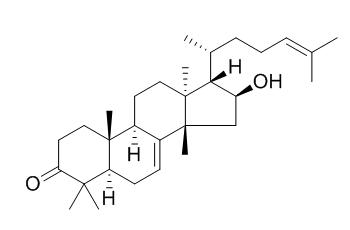
Kulinone
Kulinone has cytotoxic effects, with IC₅₀ values of 5.6-21.2 ug/mL, it inhibited (ED(50) 2.5-6.2 microg/mL) the P388 cancer cell line.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Appl Biol Chem.2021, 64(3),263?268
Pharmacognosy Magazine2017, 13(52):868-874
J Appl Biol Chem2022, 65:343−348.
Appl. Sci. 2021, 11(23),11099.
Revista Brasileira de Farmacognosia2024, 34:1091-1100.
Foods.2023, 12(7):1355.
Hortic Res.2023, 10(9):uhad154.
Nutrients2020, 12(2):488
JPC-Journal of Planar Chromatography 2017, 30(4)
Tea Res. Ins. Of China2017, 1-12
Related and Featured Products
Planta Med. 2011 Jun;77(9):922-8.
Cytotoxic triterpenoids and steroids from the bark of Melia azedarach.[Pubmed:
21243584]
METHODS AND RESULTS:
Two new triterpenoids (1, 2) and two new steroids (3, 4) along with twelve related known compounds (5-16) were isolated from the bark of Melia azedarach. The new structures were elucidated by means of spectroscopic methods and molecular modeling studies and found to be 21,24-cycloeupha-7-ene-3 β,16 β,21 α,25-tetrol (1), 3 β-acetoxy-12 β-hydroxy-eupha-7,24-dien-21,16 β-olide (2), 29-hydroperoxy-stigmasta-7,24(28) E-dien-3 β-ol (3), and 24 ξ-hydroperoxy-24-vinyl-lathosterol (4). All isolated compounds were tested for their cytotoxic activity against three human cancer cell lines (A549, H460, HGC27) using the CellTiter Glo™ luminescent cell viability assay.
CONCLUSIONS:
Among them, compounds 2- 4, 24 ξ-hydroperoxy-24-vinyl-cholesterol (6), Kulinone (7), meliastatin 3 ( 8), 3-oxo-olean-12-en-28-oic acid (10), and (22 E,24 S)-5 α,8 α-epidioxy-24-methyl-cholesta-6,22-dien-3 β-ol (12) were found to have cytotoxic effects, with IC₅₀ values of 5.6-21.2 μg/mL.
J Nat Prod. 2002 Dec;65(12):1886-91.
Antineoplastic agents. 489. Isolation and structures of meliastatins 1-5 and related euphane triterpenes from the tree Melia dubia.[Pubmed:
12502333 ]
The bark of the giant neem tree Melia dubia was found to contain 11 euphane-type triterpenes.
METHODS AND RESULTS:
Five new compounds, meliastatins 1-5 (1-5), proved to inhibit growth of the P388 lymphocytic leukemia cell line (ED(50) 1.7-5.6 microg/mL). Four of the others, the previously known methyl kulonate (8), Kulinone (9), 16-hydroxybutyrospermol (10), and kulactone (11), were also found to inhibit (ED(50) 2.5-6.2 microg/mL) the P388 cancer cell line. In addition, two new euphane triterpenes were isolated and named dubione A (6) and dubione B (7).
CONCLUSIONS:
Structures for each of the 11 euphane triterpenes were established by spectral techniques that included HRMS and 2D NMR.