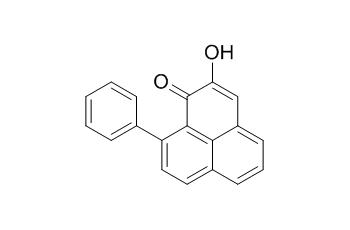
Anigorufone
Anigorufone is a phytoalexin, it shows striking antinematode activity. Anigorufone has shown inhibitory activity on the growth of the germinative tube of Fusarium oxysporum f. sp. cubense race 4.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytother Res.2022, ptr.7573.
Molecules.2019, 24(2):E343
Molecules.2021, 26(9):2791.
Chengdu University of Traditional Chinese Medicine2024, 4802935.
Anal Biochem.2019, 569:10-15
Front Pharmacol.2022, 13:919230.
Molecules.2022, 27(13):4227.
Heliyon.2024, 10(23):e40758.
Int J Mol Sci.2022, 23(24):16000.
Mediators Inflamm. 2016, 2016:6189590
Related and Featured Products
Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):105-10.
Phenalenone-type phytoalexins mediate resistance of banana plants (Musa spp.) to the burrowing nematode Radopholus similis.[Pubmed:
24324151 ]
The global yield of bananas-one of the most important food crops-is severely hampered by parasites, such as nematodes, which cause yield losses up to 75%.
METHODS AND RESULTS:
Plant-nematode interactions of two banana cultivars differing in susceptibility to Radopholus similis were investigated by combining the conventional and spatially resolved analytical techniques (1)H NMR spectroscopy, matrix-free UV-laser desorption/ionization mass spectrometric imaging, and Raman microspectroscopy. This innovative combination of analytical techniques was applied to isolate, identify, and locate the banana-specific type of phytoalexins, phenylphenalenones, in the R. similis-caused lesions of the plants.
CONCLUSIONS:
The striking antinematode activity of the phenylphenalenone Anigorufone, its ingestion by the nematode, and its subsequent localization in lipid droplets within the nematode is reported.
The importance of varying local concentrations of these specialized metabolites in infected plant tissues, their involvement in the plant's defense system, and derived strategies for improving banana resistance are highlighted.
Antimicrob Agents Chemother. 2004 May;48(5):1534-40.
Fungus-elicited metabolites from plants as an enriched source for new leishmanicidal agents: antifungal phenyl-phenalenone phytoalexins from the banana plant (Musa acuminata) target mitochondria of Leishmania donovani promastigotes.[Pubmed:
15105102 ]
METHODS AND RESULTS:
Two antifungal phenyl-phenalenone phytoalexins isolated from the banana plant (Musa acuminata) elicited with the fungus Fusarium oxysporum, together with a methoxy derivative of one of them and two epoxide precursors of their chemical synthesis, were tested for leishmanicidal activity on Leishmania donovani promastigotes and L. infantum amastigotes. Drugs inhibited proliferation of both forms of the parasite with a 50% lethal concentration range between 10.3 and 68.7 micro g/ml. Their lethal mechanism was found linked to the respiratory chain by a systematic approach, including electron microscopy, measurement of the oxygen consumption rate on digitonin-permeabilized promastigotes, and enzymatic assays on a mitochondrial enriched fraction. Whereas the whole set of compounds inhibited the activity of fumarate reductase in the mitochondrial fraction (50% effective concentration [EC(50)] between 33.3 and 78.8 micro g/ml) and on purified enzyme (EC(50) = 53.3 to 115 micro g/ml), inhibition for succinate dehydrogenase was only observed for the two phytoalexins with the highest leishmanicidal activity: Anigorufone and its natural analogue 2-methoxy-9-phenyl-phenalen-1-one (EC(50) = 33.5 and 59.6 micro g/ml, respectively).
CONCLUSIONS:
These results provided a new structural motif, phenyl-phenalenone, as a new lead for leishmanicidal activity, and support the use of plant extracts enriched in antifungal phytoalexins, synthesized under fungal challenge, as a more rational and effective strategy to screen for new plant leishmanicidal drugs.
Natural Product Letters, 1995, 6(1):23-30.
New Phenalenone-type Phytoalexins from Musa acuminata (Colla AAA) Grand Nain[Reference:
WebLink]
Four new phenalenone-type phytoalexins (2–5) have been isolated from rhizomes of Musa acuminata (AAA) infected with the fungus Fusarium oxysporum.
METHODS AND RESULTS:
The structures of the new phytoalexins were elucidated using spectroscopic evidence, chemical correlation, synthesis, and X-ray diffraction analysis. The major compound 2, has been previously described as a natural product, named Anigorufone, but never as a phytoalexia. The chemical shift for all of the hydrogen and carbon atoms in the substances were unambiguously established by mono- and bidimensional, homo- and heteronuclear NMR experiments (1HNMR, 13CNMR, COSY, HMQC and HMBC).
CONCLUSIONS:
In preliminary in vitro assays, all of the four new phytoalexins have shown inhibitory activity on the growth of the germinative tube of Fusarium oxysporum f. sp. cubense race 4.