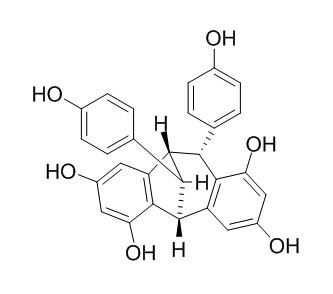
Ampelopsin F
Ampelopsin F is a natural product from Ampelopsis brevipedunculata var. hancei.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Appl Microbiol Biotechnol.2016, 100(9):3965-77
International J of Green Pharmacy2019, 13(3)
J Biosci.2020, 45:46.
Institut Pasteur Korea2020, doi: 10.21203.
Molecules.2024, 29(22):5260.
Int J Mol Sci.2023, 24(22):16465.
Food Research International2016, 106-113
Heliyon.2022, 8(12):e12031.
Antioxidants (Basel).2020, 9(2):E120
J Nat Med.2017, 71(2):380-388
Related and Featured Products
J Asian Nat Prod Res. 2014;16(11):1099-107.
A new symmetrical tetramer oligostilbenoid containing tetrahydrofuran ring from the stem bark of Dryobalanops lanceolata.[Pubmed:
25034352 ]
A new tetramer oligostilbenoid possessing tetrahydrofuran ring, malaysianol C (1), was isolated from the acetone extract of the stem bark of Dryobalanops lanceolata, together with four known oligostilbenoids nepalensinol E (2), ϵ-viniferin (3), laevifonol (4), and Ampelopsin F (5).
METHODS AND RESULTS:
The structures of isolated compounds were elucidated on the basis of spectral evidence. The antibacterial activity of the isolated compounds was evaluated using resazurin microtitre-plate assay, whereas the cytotoxic activity was tested using MTT assay. The plausible biogenetic routes of the isolated compounds are also discussed.
Bioorg Med Chem Lett. 2012 Jan 15;22(2):973-6.
Antioxidative oligostilbenes from Caragana sinica.[Pubmed:
22209460 ]
Two new oligostilbenes, caragasinins A (5) and B (10), and eight known compounds, kobophenol A (1), (+)-α-viniferin (2), (+)-Ampelopsin F (3), pallidol (4), (+)-isoAmpelopsin F (6), miyabenol C (7), carasinaurone (8) and caraphenol B (9) were isolated from the ethylacetate-soluble extract of the roots of Caragana sinica.
METHODS AND RESULTS:
The structures of the isolates were determined on the basis of extensive spectroscopic analysis including 1D, 2D NMR and HRESI-MS. These compounds were assessed for antioxidant activities. Caragasinin A (5), caraphenol B (9), and caragasinin B (10) showed moderate DPPH scavenging activity and lipid peroxidation inhibitory activities with IC(50) values ranging from 34.7±1.0 to 89.1±2.3μM.