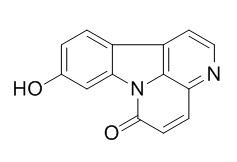
9-Hydroxycanthin-6-one
9-Hydroxycanthin-6-one might be the active component that contributed to the aphrodisiac effect of E. longifolia by antagonizing the smooth muscle tone of CC as well as SV probably through interfering with Ca2+ mobilization.9-Hydroxycanthin-6-one inhibits Wnt signaling through the activation of GSK3β independent of CK1α.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nutrients.2019, 11(11):E2694
Life (Basel).2021, 11(12):1399.
Proc. Sci. Math.2024, V25:108-123
Appl. Sci.2021, 11(19),9343.
Separations2021, 8(1), 1.
Molecules.2024, 29(23):5632.
Molecules2022, 27(9):2613.
Sains Malaysiana2022, 51(4):1143-1154
Pharmacol Rep.2017, 69(6):1224-1231
J Cell Mol Med.2024, 28(16):e70016.
Related and Featured Products
J Sex Med. 2012 Apr;9(4):1027-36.
9-hydroxycanthin-6-one induces penile erection and delays ejaculation.[Pubmed:
21569213]
Eurycoma longifolia Jack (Simaroubaceae) has the reputation as a male aphrodisiac because it is claimed to increase virility and sexual prowess. Nevertheless, whether or not E. longifolia regulates directly the muscle tone of corpus cavernosa and/or seminal vesicle (SV) remains unclear. Even until now, the compositions that could account for its aphrodisiac property are still unknown.We examined the effect of 9-Hydroxycanthin-6-one (9-HC-6-one), a β-carboline alkaloid isolated from E. longifolia, on penile erection and ejaculation, and further elucidated the mechanism of action. 9-Hydroxycanthin-6-one induces penile erection and delays ejaculation.
METHODS AND RESULTS:
Drug's effect was studied on rat corpus cavernosum (CC) and SV in vitro, and on the changes in intracavernosal pressure (ICP) after IC injection and intraluminal pressure (ILP) of the SV after hypogastric nerve stimulation (HNS), respectively. 9-Hydroxycanthin-6-one relaxed significantly phenylephrine (PE)-precontracted CC. Such response was not attenuated by endothelium disruption, N(G) -nitro-L-arginine methyl ester, or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one treatment, suggesting that a nitric oxide/cyclic guanosine monophosphate-dependent pathway was precluded. 9-Hydroxycanthin-6-one attenuated PE-induced contraction by blocking cell surface and internal calcium channels with a higher potency for internal calcium release. This compound also antagonized calcium-evoked contraction in Ca2+ -free, high K+ -depolarizing condition, suggesting that interfering with the entry of calcium through voltage-dependent channels also contributed to 9-Hydroxycanthin-6-one-induced corporal relaxation. After IC application of 9-Hydroxycanthin-6-one, a significant rise in ICP was observed as compared with the application of normal saline. 9-Hydroxycanthin-6-one relaxed significantly norepinephrine (NE)- and KCl-precontracted SV, and antagonized NE-induced oscillatory contraction as potent as clomipramine. Finally, the HNS-evoked increase in ILP was dose-dependently repressed after challenge by 9-Hydroxycanthin-6-one.
CONCLUSIONS:
9-Hydroxycanthin-6-one might be the active component that contributed to the aphrodisiac effect of E. longifolia by antagonizing the smooth muscle tone of CC as well as SV probably through interfering with Ca2+ mobilization.
J Nat Prod. 2015 May 22;78(5):1139-46.
9-Hydroxycanthin-6-one, a β-Carboline Alkaloid from Eurycoma longifolia, Is the First Wnt Signal Inhibitor through Activation of Glycogen Synthase Kinase 3β without Depending on Casein Kinase 1α.[Pubmed:
25905468]
Wnt signaling regulates various processes such as cell proliferation, differentiation, and embryo development. However, numerous diseases have been attributed to the aberrant transduction of Wnt signaling.
METHODS AND RESULTS:
We screened a plant extract library targeting TCF/β-catenin transcriptional modulating activity with a cell-based luciferase assay. Activity-guided fractionation of the MeOH extract of the E. longifolia root led to the isolation of 9-Hydroxycanthin-6-one (1). Compound 1 exhibited TCF/β-catenin inhibitory activity. Compound 1 decreased the expression of Wnt signal target genes, mitf and zic2a, in zebrafish embryos. Treatment of SW480 cells with 1 decreased β-catenin and increased phosphorylated β-catenin (Ser 33, 37, Tyr 41) protein levels. The degradation of β-catenin by 1 was suppressed by GSK3β-siRNA, while compound 1 decreased β-catenin even in the presence of CK1α-siRNA.
CONCLUSIONS:
These results suggest that 1 inhibits Wnt signaling through the activation of GSK3β independent of CK1α.
J Nat Prod. 1991 Sep-Oct;54(5):1360-7.
Cytotoxic and antimalarial constituents of the roots of Eurycoma longifolia.[Pubmed:
1800638]
By bioactivity-directed fractionation, five cytotoxic constituents have been characterized from the roots of Eurycoma longifolia collected in Kalimantan, Indonesia.
METHODS AND RESULTS:
Four canthin-6-one alkaloids, namely, 9-methoxycanthin-6-one, 9-methoxycanthin-6-one-N-oxide, 9-Hydroxycanthin-6-one, and 9-Hydroxycanthin-6-one-N-oxide, and one quassinoid, eurycomanone, were found to be cytotoxic principles. Each of these compounds was evaluated against a panel of cell lines comprising a number of human cancer cell types [breast, colon, fibrosarcoma, lung, melanoma, KB, and KB-V1 (a multi-drug resistant cell line derived from KB)] and murine lymphocytic leukemia (P-388).
CONCLUSIONS:
The canthin-6-ones 1-4 were found to be active with all cell lines tested except for the KB-V1 cell line. Eurycomanone was inactive against murine lymphocytic leukemia (P-388) but was significantly active against the human cell lines tested. Two additional isolates, the beta-carboline alkaloids beta-carboline-1-propionic acid and 7-methoxy-beta-carboline-1-propionic acid, were not significantly active with these cultured cells. However, compounds 5 and 7 were found to demonstrate significant antimalarial activity as judged by studies conducted with cultured Plasmodium falciparum strains. The structures of the novel compounds 2-4 and 7 were established by spectral and chemical methods.