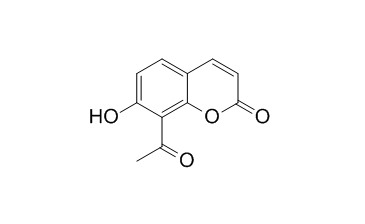
8-Acetyl-7-hydroxycoumarin shows an interesting activity against V. cholerae, a key bacterium in the contaminated water.
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Acta Pharmaceutica Sinica, 2008, 43(9):930-933.
Synthesis and antinociceptive activity of seselin derivatives.[Reference:
WebLink]
METHODS AND RESULTS:
Natural product seselin and related derivatives with an angular pyranocoumarin skeleton were synthesized from 8-Acetyl-7-hydroxycoumarins by condensation with acetone,reduction,and dehydration successively under mild conditions with total yield of 50%.Twelve seselin derivatives were tested by the writhing response assay induced by acetic acid at a dose of 40 mg·kg~(-1).
CONCLUSIONS:
Seselin(4a) and 4,8,8-trimethyl-9,9-dihydro-pyranchromene-2,10-dione(2b) showed obviously antinociceptive activity with inhibitory effect of 85% and 50%,respectively,more or quite potent than aspirin in the same assay,suggesting that seselin derivatives could be a novel kind of potential antinociceptive agents.
Journal of Agricultural and Food Chemistry, 2006,54(10):3521-3527.
Antifungal and Antibacterial Activities of Mexican Tarragon (Tagetes lucida).[Reference:
WebLink]
Mexican tarragon (Tagetes lucida Cv. Asteraceae: Campanulatae) is an important, nutritious plant and an effective herbal medicine.
METHODS AND RESULTS:
Seven coumarins, 7,8-dihydroxycoumarin (4), umbelliferone (7-hydroxycoumarin) (5), scoparone (6,7-dimethoxycoumarin) (7), esculetin (6,7-dihydroxycoumarin) (11), 6-hydroxy-7-methoxycoumarin (12), herniarin (7-methoxycoumarin) (13), and scopoletin (6-methoxy-7-hydroxycoumarin) (14), and three flavonoids, patuletin (18), quercetin (19), and quercetagetin (20), were isolated from CH2Cl2 and MeOH extracts from aerial parts of T. lucida. In addition, 6,7-diacetoxy coumarin (15), 6-methoxy-7-acetylcoumarin (16), and 6-acetoxy-7-methoxycoumarin (17) derivatives were synthesized. 8-Methoxypsoralen (1), 8-Acetyl-7-hydroxycoumarin (2), 7,8-dihydroxy-6-meth-oxycoumarin (3), 6,7-dimethoxy-4-methylcoumarin (6), 5,7-dihydroxy-4-methylcoumarin (8), 4-hydroxycoumarin (9), 4-hydroxy-6,7-dimethylcoumarin (10), naringenin (21), glycoside-7-rhamnonaringin (22), and rutin (23) were commercially obtained (Sigma-Aldrich). All of these compounds and extracts (M1 and M2) were assayed against bacteria and fungi. The antibacterial activity was determined on Bacillus subtilis, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Salmonella typhi, Salmonella sp., Shigella boydii, Shigella sp., Enterobacter aerogenes, Enterobacter agglomerans, Sarcina lutea, Staphylococcus epidermidis, Staphylococcus aureus, Yersinia enterolitica, Vibrio cholerae (three El Tor strains, CDC-V12, clinic case, and INDRE-206, were obtained from contaminated water), and V. cholerae (NO-O1). The evaluated fungi were Aspergillus niger, Penicillium notatum, Fusarium moniliforme, Fusarium sporotrichum, Rhizoctonia solani, and Trichophyton mentagrophytes.
CONCLUSIONS:
The most active compounds against Gram-positive and -negative bacteria were the dihydroxylated coumarins 3 and 4. In addition, 2-4, 6, 7, and 11 showed an interesting activity against V. cholerae, a key bacterium in the contaminated water; 2-4 were the most active. Coumarins were the most effective compounds against Gram-negative bacteria. The extract MeOH/CH2Cl2 (1: 4) M2 at 0.4 microg/disk inhibited the growth of E. coli and P. mirabilis (40%), K. pneumoniae (31.1%), Salmonella sp. (35.5%), and Shigella sp. (0%) at 72 h of culture. The dimethoxy compounds 6 and 7 showed a strong activity against fungal strains, especially T. mentagrophytes and R. solani (100% of inhibition at 125.0 and 250.0 microg/mL, respectively).
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2009, 73(1):35-40.
Probing the binding of 8-Acetyl-7-hydroxycoumarin to human serum albumin by spectroscopic methods and molecular modeling.[Reference:
WebLink]
METHODS AND RESULTS:
Interaction of 8-Acetyl-7-hydroxycoumarin with human serum albumin (HSA) at pH 7.40 has been investigated at 291, 301 and 310 K, respectively, employing the steady fluorescence, circular dichroism (CD) and molecular modeling methods.
The quenching mechanism and binding constants were determined by the fluorescence quenching experiments. Thermodynamic data showed that 8-Acetyl-7-hydroxycoumarin was included in the hydrophobic cavity of HSA via hydrophobic interactions. The result of CD indicated that the binding of 8-Acetyl-7-hydroxycoumarin to HSA causes a slight conformational change of the protein. Furthermore, upon binding with HSA, the fluorescence spectra of the 8-Acetyl-7-hydroxycoumarin exhibits appreciable hypsochromic shift associated with an enhancement in the fluorescence intensity. The binding constant (K) and the standard free energy change (ΔG0) have been also calculated according to the fluorescence data of the ligand, which is in good agreement with the values determined by fluorescence quenching data of HSA.
CONCLUSIONS:
Computational mapping of the possible binding sites of 8-Acetyl-7-hydroxycoumarin revealed that the molecule was bound in the large hydrophobic cavity of subdomain IIA mainly by the hydrophobic interaction and also by the hydrogen bonding interactions between 8-Acetyl-7-hydroxycoumarin and the residues His 242, Arg 222, and Arg 218.