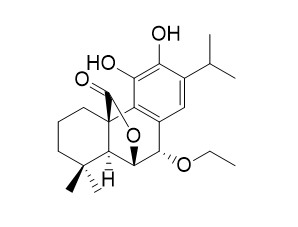
7-Ethoxyrosmanol
7-Ethoxyrosmanol has cytotoxic and antimicrobial activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2015, 22(14):1262-8
Molecules. 2013, 18(7):7376-88
Mol Biol Rep.2024, 51(1):117.
Acta Pharmaceutica Hungarica2016, 86:35-40
Hindawi J of Food Biochemistry2023, P17:8883860
J Biomol Struct Dyn.2022, 5;1-17.
Am J Chin Med.2016, 44(8):1719-1735
Int J Mol Sci.2023, 24(8):7442.
Vet World.2023, 16(3):618-630.
The Journal of Supercritical Fluids2021, 176:105305.
Related and Featured Products
Current Bioactive Compounds, 2015, 11(3):-.
Cytotoxic and Antimicrobial Diterpenes Isolated from Hyptis Dilatata.[Reference:
WebLink]
Diterpenes 7-Ethoxyrosmanol (1) and carnosol (2) were isolated and identified from the extracts of Hyptis dilatata collected in the Orinoco region, Colombia, used traditionally in this region for the treatment of wound infections of cattle. Their stereostructures were elucidated by ID and 2D NMR experiments including 1H, 13C, COSY, HSQC, HMBC and NOESY. Although compound 1 has been reported previously, its NMR assignments were revised, completed and corrected. These compounds showed a specific antimicrobial activity against Gram (+) strains.
METHODS AND RESULTS:
The cytotoxic activity of compounds 1 and 2 was evaluated against a panel of three cancer cell lines (MCF7, HeLa and HT29) using curcumin as positive control. Carnosol (2) showed cytotoxic activity against all three cell lines having a similar response as the positive control with an IC50 approximately of 15-20 μg/mL in each case. On the other hand, 7-Ethoxyrosmanol (1) showed lower activity than carnosol, with an IC50 approx. of 20-30 μg/mL for the three cancer cell lines. This is the first report on cytotoxic activity against MCF7, HeLa and HT29 cancer cell lines and the antimicrobial activity of compound 1, and the first report on cytotoxic activity against HeLa of compound 2. In addition, the main volatile components of the essential oil of H. dilatata which displayed quorum sensing inhibitory activity were identified by GC/MS analysis.
CONCLUSIONS:
Finally, these results let us to propose H. dilatata as a potential resource of antimicrobial and cytotoxic compounds.
Anticancer research, 2012, 32(11):4781-4789.
Phenolic diterpenes derived from Hyptis incana induce apoptosis and G(2)/M arrest of neuroblastoma cells.[Pubmed:
23155243]
Neuroblastoma is one of the most commonly encountered solid tumors in the pediatric age group, and the prognosis of patients with advanced neuroblastoma is very poor. In this study, the antitumor effects of five phenolic diterpenes derived from Hyptis incana (Lamiaceae), a Brazilian medicinal plant, were examined on neuroblastoma cells.
METHODS AND RESULTS:
Cytotoxicity was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Apoptotic nuclear shrinkage was monitored by Hoechst 33342 staining. The cell-cycle status was evaluated by flow cytometry and protein alterations were monitored by western blotting. Differentiated cells were photographed and counted in a randomized fashion. All of the examined compounds exhibited significant cytotoxicity towards the neuroblastoma cells. In particular, 7-Ethoxyrosmanol had a high degree of efficacy. Nuclear condensation and degradation of procaspase-3 and -9 were observed after treatment of the cells with these compounds. Moreover, phenolic diterpenes induced cell-cycle arrest in the G(2)/M phase. Rosmanol and epirosmanol tended to induce differentiation.
CONCLUSIONS:
Phenolic diterpenes isolated from H. incana have multiple antitumor effects on neuroblastoma cells.
Journal of Natural Products, 2002, 65(7):986-989.
Semisynthesis of Rosmanol and Its Derivatives. Easy Access to AbietatrieneDiterpenes Isolated from the Genus Salvia with Biological Activities.[Pubmed:
12141857]
METHODS AND RESULTS:
The known diterpenes rosmanol (3), rosmaquinone (4), 7-methoxyrosmanol (5), 7-Ethoxyrosmanol (6), galdosol (7), and epirosmanol (8) have been obtained by partial synthesis from carnosol (2), an abundant natural product present in Salvia species. The physical and spectroscopic data of these semisynthetic diterpenes were identical to those of authentic natural samples and with data reported in the literature. These abietane diterpenes have very interesting biological activities and are present in the genus Salvia in low quantities; thus, the semisynthetic approach described here represents an efficient alternative method to obtain these compounds.
CONCLUSIONS:
Additionally, the known diterpene 16-hydroxyrosmanol (10) and a new aromatic diterpene 11 were obtained from 16-hydroxycarnosol (9) by reaction with Ph3P/NBS in CH2Cl2. The structure of the new compound 11 was established from its spectroscopic data as 12,16-epoxycarnosol.