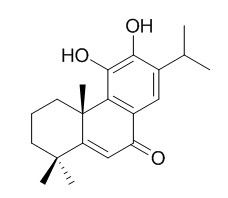
Salvinolone
Salvinolone, demethyl cryptojaponol and taxodione show potent activity with 4-10 microg/mL of MIC against MRSA and 4-16 microg/mL of MIC against VRE.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2017, 22(2)
Pharmaceuticals (Basel).2021, 14(8):742.
Phytomedicine.2024, 155760.
Phytomedicine.2019, 67:153159
Biochem Pharmacol. 2020, 177:114014.
Tropical J. of Pha. Research2017, 16(3):543-552
Front Pharmacol.2022, 13:906763.
Translational Neuroscience2024, 15:20220339
Journal of Ginseng Research2022, j.jgr.2022.09.005.
Korean J. of Horticultural Sci. & Tech. 2017, 793-804
Related and Featured Products
J Chem Ecol. 2009 Jun;35(6):635-42.
Antitermitic activities of abietane-type diterpenes from Taxodium distichum cones.[Pubmed:
19475449]
METHODS AND RESULTS:
Eight known abietane-type diterpenes were isolated from the weak acidic fraction of the n-hexane extract from cones of Taxodium distichum, one of the extant, living fossil conifers. They were identified as 6,7-dehydroroyleanone (1), taxodal (2), taxodione (3), Salvinolone (4), 14-deoxycoleon U (5), 5,6-dehydrosugiol (6), sandaracopimaric acid (7), and xanthoperol (8). The structures of these compounds were determined by comparison of NMR spectral data with published data. The antitermitic (termicidal and antifeedant) activities of the compounds 1-8 against the subterranean termite, Reticulitermes speratus Kolbe, were evaluated.
CONCLUSIONS:
Compounds 1 and 3 showed potent termicidal activity, and 5 and 8 showed potent antifeedant activity. Compound 1 was found to be one of the representative bioactive compounds in the n-hexane extract of T. distichum cones. Compounds 1-8, with the exception of 7, were oxides of ferruginol (9). Therefore, the presence of various oxidation forms of the abietane-type structure reflects their various bioactivities.
Bioorg Med Chem. 2001 Feb;9(2):347-56.
Synthesis of variously oxidized abietane diterpenes and their antibacterial activities against MRSA and VRE.[Pubmed:
11249127]
METHODS AND RESULTS:
Variously oxidized 12 natural abietanes, 6,7-dehydroferruginol methyl ether (3), ferruginol (5), 11-hydroxy-12-oxo-7,9(11),13-abietatriene (7), royleanone (9), demethyl cryptojaponol (12), Salvinolone (14), sugiol methyl ether (16), sugiol (17), 5,6-dehydrosugiol methyl ether (19), 5,6-dehydrosugiol (20), 6beta-hydroxyferruginol (23), and taxodione (25) were synthesized. Antimicrobial activities of synthesized phenolic diterpenes and their related compounds against MRSA and VRE were evaluated.
CONCLUSIONS:
Phenols (12-hydroxyabieta-8,11,13-trien-6-one 22 and 23), catechols (12 and 14) and taxodione 25 showed potent activity with 4-10 microg/mL of MIC against MRSA and 4-16 microg/mL of MIC against VRE. (-)-Ferruginol showed more potent activity than natural type (+)-ferruginol. Quinone methide 7 showed the most potent activity with 0.5-1 microg/mL of MIC against both MRSA and VRE.
Nat Prod Commun. 2011 Jan;6(1):3-5.
Bioactive diterpenes from Clerodendrum kaichianum.[Pubmed:
21366034]
METHODS AND RESULTS:
Bioassay-guided isolation studies of the extract of Clerodendrum kaichianum Hsu., a new rearranged abietane diterpene and five known diterpene compounds were isolated by various chromatography methods. Their structures were identified by means of spectroscopic methods, including 1D- and 2D-NMR spectroscopy, as (16R)-12,16-epoxy-11,14,17-trihydroxy-17(15-->16)-abeo-8,11,13-abietatrien-7-one (1), villosin A (2), Salvinolone (3), 14-deoxyloleon U (4), 5,6-dehydrosugiol (5), and coleon U (6).
CONCLUSIONS:
Compounds 1, 2, 3, and 5 are reported for the first time for this genus. Compounds 1, 2, and 6 demonstrated potent cytotoxic activities against the HL-60 tumor cell line.