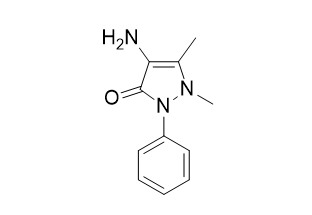
4-Aminoantipyrine
4-Aminoantipyrine as an inhibitor of mild steel corrosion in HCl solution. It used as an intermediate for the synthesis of pharmaceuticals especially antipyretic and analgesic drugs. 4-Aminoantipyrine reduces toxic and genotoxic effects of doxorubicin, cisplatin, and cyclophosphamide in male mice.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2015, 20(10):19172-88
J Korean Soc Food Sci Nutr2023, 52(7): 750-757
Cells.2021, 10(11):2919.
J Neuroinflammation.2020, 17(1):75.
ACS Pharmacol. Transl. Sci.2023, 3c00129.
Molecules.2018, 23(2)
Research Square2021, 10.21203.
Antiviral Res.2013, 98(3):386-93
Int J Mol Sci.2017, 19(1)
Int J Biol Macromol.2020, 169:342-351
Related and Featured Products
Journal of Applied Electrochemistry, 1999, 29(5):593-599.
4-Aminoantipyrine as an inhibitor of mild steel corrosion in HCl solution.[Reference:
WebLink]
METHODS AND RESULTS:
4-Aminoantipyrine (AAP) was tested as a corrosion inhibitor for mild steel in 2 M HCl solution using different techniques: weight loss, potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). The results showed that AAP is an inhibitor for mild steel in this medium. The inhibition was assumed to occur via adsorption of the inhibitor molecule on the metal surface. In the 20 to 60 ∘C temperature range, the AAP adsorption follows the Flory–Huggins isotherm and/or the El-Awady et al. kinetic-thermodynamic model.
CONCLUSIONS:
The protection efficiency increases with increasing inhibitor concentration (in the range 10−310−2 M) but decreases with increasing temperature. The thermodynamic functions of dissolution and adsorption processes were calculated.
Journal of hazardous materials, 2011, 192(3):1766-1771.
Effect of 4-aminoantipyrine on oxidative stress induced by glutathione depletion in single human erythrocytes using a microfluidic device together with fluorescence imaging.[Reference:
WebLink]
METHODS AND RESULTS:
The effects of 4-Aminoantipyrine (AAP) on oxidative stress induced by glutathione (GSH) depletion in single human erythrocytes were investigated using microfluidic technique and fluorescence imaging. Most cell-based toxicity evaluations on GSH are performed with bulk experiments based on analysis of cell populations. This work established a single-cell toxicity evaluation method to statistically analyze the GSH amount in single erythrocytes incubated with AAP in different concentrations. The experimental conditions of cell flow rate and cell concentration were optimized. The GSH contents in erythrocytes decreased with increasing dose of AAP. At low concentration, AAP had a little effect on GSH; while at high concentration, AAP led to GSH depletion reaching a maximum of 14.53%. The depletion of GSH leads to a significant shift to a more oxidizing intracellular environment.
CONCLUSIONS:
This study provides basic data for presenting the effect of AAP on GSH in erythrocytes and is helpful for understanding its toxicity during the blood transportation process. In addition, it will also complement studies on the environmental risk assessment of AAP pollution.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2009.
Vibrational spectroscopic studies and DFT calculations of 4-aminoantipyrine.[Reference:
WebLink]
METHODS AND RESULTS:
The pyrazole derivative, 4-Aminoantipyrine (4AAP), used as an intermediate for the synthesis of pharmaceuticals especially antipyretic and analgesic drugs has been analyzed experimentally and theoretically for its vibrational frequencies. The FTIR and FT Raman spectra of the title compound have been compared with the theoretically computed frequencies invoking the standard 6-311g(d,p) and cc-pVDZ basis sets at DFT level of theory (B3LYP). The harmonic vibrational frequencies at B3LYP/cc-pVDZ after appropriate scaling method seem to coincide satisfactorily with the experimental observations rather than B3LYP/6-311g(d,p) results.
CONCLUSIONS:
The theoretical spectrograms for FT-IR and FT-Raman spectra of 4AAP have been also constructed and compared with the experimental spectra. Additionally, thermodynamic data have also been calculated and discussed.