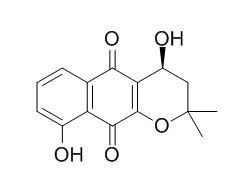
4,9-Dihydroxy-alpha-lapachone
4,9-Dihydroxy-alpha-lapachone has antitumor-promoting effects, it exhibits significant inhibitory activity against 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced Epstein-Barr virus early antigen (EBV-EA) activation in Raji cells. 4,9-Dihydroxy-alpha-lapachone exhibits potent inhibitory effects on lipopolysaccharide-induced NO synthesis in RAW 264.7 cells , with IC(50) value of 2.73 microM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Agric Food Chem.2016, 64(35):6783-90
Eur J Pharmacol.2018, 832:96-103
Appl. Sci. 2021, 11(8),3437.
Materials Today Communications2023, 37:107216
Exp Mol Med.2020, 52(4):629-642.
Phytomedicine.2019, 59:152785
Clin Transl Med.2021, 11(5):e392.
Phytomedicine.2021, 83:153483.
J Chromatogr Sci.2015, 53(5):824-9
Brain Res Bull.2024, 218:111103.
Related and Featured Products
Arch Pharm Res. 2010 Mar;33(3):381-5.
Naphthoquinones from Catalpa ovata and their inhibitory effects on the production of nitric oxide.[Pubmed:
20361302]
METHODS AND RESULTS:
Together with nine known compounds, catalponol (1), catalponone (2), catalpalactone (3), alpha-lapachone (4), 9-hydroxy-alpha-lapachone (5), 4,9-Dihydroxy-alpha-lapachone (6), 9-methoxy-alpha-lapachone (7), 4-oxo-alpha-lapachone (8), and 9-methoxy-4-oxo-alpha-lapachone (9). The structures were elucidated on the basis of spectroscopic analyses. The inhibitory effects of these isolates on lipopolysaccharide-induced NO synthesis in RAW 264.7 cells were evaluated.
CONCLUSIONS:
Among them, catapalactone (3), 9-hydroxy-alpha-lapachone (5) and 4,9-Dihydroxy-alpha-lapachone (6) exhibited potent inhibitory effects, with IC(50) values of 9.80, 4.64 and 2.73 microM, respectively.
J Nat Prod. 1998 May;61(5):629-32.
Antitumor-promoting naphthoquinones from Catalpa ovata.[Pubmed:
9599262]
METHODS AND RESULTS:
Bioassay-directed fractionation of an extract of the stem-bark of Catalpa ovata led to the isolation of three new naphthoquinones:
8-methoxydehydroiso-alpha-lapachone (1), 9-methoxy-4-oxo-alpha-lapachone (2), and (4S,4aR,10R,10aR)-4, 10-dihydroxy-2,2-dimethyl-2,3,4,4alpha,10, 10alpha-hexahydrobenzo[g]chromen-5-one (3), which is a 1,4-reductive form of 6. The known compounds 3-hydroxydehydroiso-alpha-lapachone (4), 4,9-Dihydroxy-alpha-lapachone (5), 4-hydroxy-alpha-lapachone (6), and 9-methoxy-alpha-lapachone (7), and catalpalactone (8) were also isolated. Their structures were elucidated by spectral methods.
CONCLUSIONS:
These compounds all exhibited significant inhibitory activity against 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced Epstein-Barr virus early antigen (EBV-EA) activation in Raji cells.