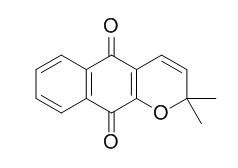
Dehydro-alpha-lapachone
Dehydro-alpha-lapachone is an antifungal substance. Dehydro-α-lapachone can inhibit vessel regeneration, interfere with vessel anastomosis, and limit plexus formation in zebrafish, it also can induce vascular pruning and growth delay in orthotopic mammary tumors in mice.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2023, 28(13):4972.
Cell Physiol Biochem.2017, 43(4):1425-1435
University of East Anglia2023, 93969.
Phytochemistry Letters2015, 243-247
Korean Journal of Pharmacognosy2019, 50(4):285-290
J. Essential Oil Research2024, 6:36:554-565.
Nutr Cancer.2023, 75(1):376-387.
Int J Mol Sci.2024, 25(1):616.
Front Pharmacol.2021, 12:744624.
Fitoterapia.2022, 157:105130.
Related and Featured Products
Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11596-601.
Dehydro-alpha-lapachone, a plant product with antivascular activity.[Pubmed:
21709229]
Antivascular agents have become a standard of treatment for many malignancies. However, most of them target the VEGF pathway and lead to refractoriness.
METHODS AND RESULTS:
To improve the diversity of options for antivascular therapy, we applied a high-throughput screen for small molecules targeting cell adhesion. We then assayed the resulting antiadhesion hits in a transgenic zebrafish line with endothelial expression of EGFP (Tg(fli1:EGFP)(y1)) to identify nontoxic molecules with antivascular activity selective to neovasculature. This screen identified Dehydro-alpha-lapachone (DAL), a natural plant product. We found that Dehydro-alpha-lapachone inhibits vessel regeneration, interferes with vessel anastomosis, and limits plexus formation in zebrafish. Furthermore, Dehydro-alpha-lapachone induces vascular pruning and growth delay in orthotopic mammary tumors in mice.
CONCLUSIONS:
We show that Dehydro-alpha-lapachone targets cell adhesion by promoting ubiquitination of the Rho-GTPase Rac1, which is frequently up-regulated in many different cancers.
Pest Manag Sci. 2006 May;62(5):414-8.
Dehydro-alpha-lapachone isolated from Catalpa ovata stems: activity against plant pathogenic fungi.[Pubmed:
16550502]
METHODS AND RESULTS:
The methanol extract of stems of Catalpa ovata G Don exhibits potent in vivo antifungal activity against Magnaporthe grisea (Hebert) Barr (rice blast) on rice plants, Botrytis cinerea Pers ex Fr (tomato grey mould) and Phytophthora infestans (Mont) de Bary (tomato late blight) on tomato plants, Puccinia recondita Rob ex Desm (wheat leaf rust) on wheat plants and Blumeria graminis (DC) Speer f. sp. hordei Marchal (barley powdery mildew) on barley plants.
CONCLUSIONS:
An antifungal substance was isolated and identified as Dehydro-alpha-lapachone from mass and nuclear magnetic resonance spectral data. It completely inhibited the mycelial growth of B. cinerea, Colletotrichum acutatum Simmonds, Colletotrichum gloeosporioides Simmonds, M. grisea and Pythium ultimum Trow over a range of 0.4-33.3 mg litre(-1). It also controlled the development of rice blast, tomato late blight, wheat leaf rust, barley powdery mildew and red pepper anthracnose (Colletotrichum coccodes (Wallr) S Hughes).
The chemical was particularly effective in suppressing red pepper anthracnose by 95% at a concentration of 125 mg litre(-1).
Appl Environ Microbiol. 1979 Aug;38(2):311-3.
Microbial transformations of natural antitumor agents: conversion of lapachol to dehydro-alpha-lapachone by Curvularia lunata.[Pubmed:
574750]
METHODS AND RESULTS:
Microbial transformation of lapachol, a naturally occurring naphthoquinone, was carried out by Curvularia lunata (NRRL 2178). The fungus brings about oxidative cyclization of the substrate to Dehydro-alpha-lapachone, which was isolated and characterized by nuclear magnetic resonance and mass spectral analyses; its structure was verified by chemical synthesis.
CONCLUSIONS:
The metabolite is a naturally occurring chromene possessing antibacterial and antitumor activities.
Lett Appl Microbiol. 2014 Jul;59(1):108-14.
Cytotoxicity of lapachol metabolites produced by probiotics.[Pubmed:
24635204]
Probiotics are currently added to a variety of functional foods to provide health benefits to the host and are commonly used by patients with gastrointestinal complaints or diseases. The therapeutic effects of lapachol continue to inspire studies to obtain derivatives with improved bioactivity and lower unwanted effects.
Therefore, the general goal of this study was to show that probiotics are able to convert lapachol and are important to assess the effects of bacterial metabolism on drug performance and toxicity.
METHODS AND RESULTS:
The microbial transformations of lapachol were carried out by Bifidobacterium sp. and Lactobacillus acidophilus and different metabolites were produced in mixed and isolated cultures. The cytotoxic activities against breast cancer and normal fibroblast cell lines of the isolated metabolites (4α-hydroxy-2,2-dimethyl-5-oxo-2,3,4,4α,5,9β-hexahydroindeno[1,2-β]pyran-9β-carboxilic acid, a new metabolite produced by mixed culture and dehydro-α-lapachone produced by isolated cultures) were assessed and compared with those of lapachol. The new metabolite displayed a lower activity against a breast cancer cell line (IC50 = 532.7 μmol l(-1) ) than lapachol (IC50 = 72.3 μmol l(-1) ), while Dehydro-alpha-lapachone (IC50 = 10.4 μmol l(-1) ) displayed a higher activity than lapachol. The present study is the first to demonstrate that probiotics are capable of converting lapachol into the most effective cytotoxic compound against a breast cancer cell line.
CONCLUSIONS:
Probiotics have been used in dairy products to promote human health and have the ability to metabolize drugs and other xenobiotics. Naphthoquinones, such as lapachol, are considered privileged scaffolds due to their high propensity to interact with biological targets. The present study is the first to demonstrate that probiotics are capable of converting lapachol into the most effective cytotoxic compound against a breast cancer cell line. The developed approach highlights the importance of probiotics to assess the effects of bacterial metabolism on drug performance and toxicity.