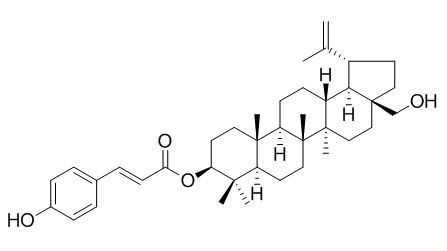
3-β-O-trans-p-coumaroylbetulinol has anti-angiogenic effect, it possesses significant anti-proliferative activity with 66.7% inhibition at 100uM.
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chem Pharm Bull (Tokyo). 2003 Nov;51(11):1318-21.
Antitumor-promoting constituents from Chaenomeles sinensis KOEHNE and their activities in JB6 mouse epidermal cells.[Pubmed:
14600382]
METHODS AND RESULTS:
Primary screening of antitumor-promoting activity using soft agar colony assays with JB6 cells was employed to isolate 22 compounds from Chaenomeles sinensis KOEHNE. These compounds were lyoniresinol-2a-O-alpha-L-rhamnopyranoside (1), lyoniresinol-2a-O-beta-D-glucopyranoside (2), aviculin (3), betulinic acid (4), betulin (5), 3-O-(E)-p-Coumaroylbetulin (6), 3-O-(E)-caffeoylbetulin (7), 3-O-(Z)-p-coumaroylbetulin (8), 3-O-(E)-caffeoyllupeol (9), alphitolic acid (10), sorbikortal II (11), tormentic acid (12), euscaphic acid (13), corosolic acid (14), maslinic acid (15), erythrodiol (16), 1-beta-D-glucopyranosyloxy-3,4,5-trimethoxybenzene (17), avicularin (18), 7-O-beta-D-glucopyranosylkaempferol (19), 5-O-beta-D-glucopyranosylgenistein (20), 7-O-beta-D-glucopyranosylgenistein (21), epicatechin (22), and beta-sitosterol (23) and were identified using spectral data such as MS, (1)H- and (13)C-NMR.
CONCLUSIONS:
Compound 1, having a rhamnosyl group, showed greater activity than 2, having a glucosyl group, and 3, which was a bis-demethoxy derivative of 1. Betulinic acid (4), having a C-28 carboxyl group, 3-O-(E)-caffeoylbetulin (7), and tormentic acid (12) showed more potent activity than betulin (5), which has a C-28 hydroxymethyl group.