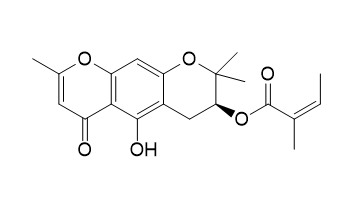
3'-O-Angeloylhamaudol
3'-O-Angeloylhamaudol has anti-inflammatory activity. 3'-O-Angeloylhamaudol induces apoptosis through the mitochondial-dependent apoptotic pathway.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Recent Pat Anticancer Drug Discov.2022, 17(4):416-426.
Exp Ther Med.2019, 18(6):4388-4396
Vietnam J. Chem.2023, 61(3),308-317
National Natural Science Foundation of China2024, pages 33.
Antioxidants (Basel).2024, 13(3):340.
Vietnam Journal of Food Control.2022, 5(3):pp.488-497.
J Appl Biol Chem2021, 64(3):245-251.
Molecules. 2013, 18(7):7376-88
Plants.2024, 13(10):1348;
Mediators Inflamm.2016, 2016:7216912
Related and Featured Products
Nat Prod Commun . 2017 Feb;12(2):255-258.
Chemical Constituents of the Roots and Rhizomes of Saposhnikovia divaricata and their Cytotoxic Activity[Pubmed:
30428224]
Phytochemical investigation of the MeOH extract of the roots and rhizomes of Saposhnikovia divaricata (Umbelliferae) resulted in the isolation of six chromons (1-6)-and five polyacetylene derivatives (7-11). Compounds 9 and 11 were isolated from S. divaricate for the first time. The chromon derivatives -(1-6) were evaluated for their cytotoxic activity against HL-60 human promyclocytic leukemia cells. Compound 1 (3'-O-Angeloylhamaudol) showed the most potent cytotoxic activity with an IC₅₀ value of 4.41 μM and was found to induce apoptotic cell death in HL-60 cells. The loss of mitochondrial membrane potential, release of cytochrome c into the cytoplasm, and activation of caspase-9 in the 1-treated HL-60 cells suggests that I induces apoptosis through the mitochondial-dependent apoptotic pathway.
Planta Med . 2011 Sep;77(13):1531-1535.
Intestinal permeability of the constituents from the roots of Saposhnikovia divaricata in the human Caco-2 cell monolayer model[Pubmed:
21308612]
The bidirectional intestinal permeability of the active constituents from the roots of Saposhnikovia divaricata, including four coumarins, anomalin (1), 5-methoxy-7-(3,3-dimethylallyloxy)coumarin (2), decursin (3), and decursinol angelate (4), as well as four chromones, cimifugin (5), prim-O-glucosylcimifugin (6), 3'- O-angeloylhamaudol (7), and sec-O-glucosylhamaudol (8), was studied by using the Caco-2 cell monolayer. These compounds were assayed by HPLC, and their transport parameters, including apparent permeability coefficients (P(app)), were then calculated. The bidirectional P(app) values of the compounds were compared with those of the markers, propranolol and atenolol. Compounds 1-5 and 7 were assigned to well-absorbed compounds, while 6 and 8 were assigned to moderately absorbed compounds. The transport of 1-7 increased linearly as a function of time up to 180 min and concentration within the test range of 10-200 μM, thus their passive diffusion mechanism was proposed. The results provided some useful information for predicting the intestinal absorption in vivo of these compounds.
J Nat Med. 2016 Apr;70(2):253-259.
Comparative analysis of the constituents in Saposhnikoviae Radix and Glehniae Radix cum Rhizoma by monitoring inhibitory activity of nitric oxide production[Pubmed:
26833192]
During the development of natural herbal medicines in Japan, Glehniae Radix cum Rhizoma (Hamabofu in Japanese) has been used as a substitute for Saposhnikoviae Radix (Bofu). Bofu and Hamabofu are blended differently in several Kampo formulae. For example, Bofu is included in Jumihaidokuto by a manufacturer, whereas Hamabofu is included instead of Bofu in the same formula by other manufacturers. Although both Bofu and Hamabofu are used for their expected anti-inflammatory effects, differences in their medicinal properties are not well characterized. In addition, there have been very few reports comparing the pharmacological activities of the constituents in Bofu and Hamabofu. In the present study, we investigated the anti-inflammatory effects of the extracts of Bofu and Hamabofu by monitoring levels of the inflammatory mediator nitric oxide (NO) produced in rat hepatocytes. Moreover, the chemical constituents responsible for the activity were investigated. Our results showed that ethyl acetate fractions of Bofu and Hamabofu extracts contain different compounds, although both fractions suppressed NO production in rat hepatocytes. The linear dihydropyranochromones from the Bofu extract (i.e., 3'-O-Angeloylhamaudol, ledebouriellol and hamaudol) suppressed NO production, whereas the coumarins from the Hamabofu extract (i.e., umbelliferone and scopoletin) also suppressed NO production. These results suggest that linear dihydropyranochromones and coumarins are responsible for the anti-inflammatory effects of Bofu and Hamabofu. It is plausible that Bofu and Hamabofu are blended differently in several Kampo formulae due to many constituents with as yet unidentified pharmacological activity.
Zhongguo Zhong Yao Za Zhi . 2010 Jun;35(12):1569-72.
[Chemical constituents of roots of Saposhnikovia divaricata][Pubmed:
20815209]
Objective: To study the chemical constituents in the dried roots of Saposhnikovia divaricata.
Method: The chemical constituents were isolated by various column chromatographic methods and structurally elucidated by IR, UV, MS and NMR evidences.
Result: Eighteen compounds were obtained and identified as 3'-O-Angeloylhamaudol (1), isobergapten (2), imperatorin (3), pentacosane acid (4), anomalin (5), decursin (6), 5-methoxy-7-(3,3-dimethylallyl- oxy)coumarin (7), decursinol angelate (8), xanthotoxin (9), bergapten (10), tectochrysin (11), scopoletin (12), hamaudol (13), ledebouriellol (14), cimifugin (15), sec-O-glucosylhamaudol (16), 4'-O-beta-D-glucosyl-5-O-methylvisamminol (17), and prim-O-glucosylcimifugin (18).
Conclusion: Compounds 2, 6-8, and 11 were isolated from the roots of S. divaricata for the first time. Compounds 1 and 13-18 were chromones, 2, 3, 5-10 and 12 were coumarins, 4 was fatty acid, and 11 was flavonoid.