Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Academic Journal of Second Military Medical University, 2010, 30(7):760-763.
RRLC-TOF/MS in identification of constituents and metabolites of Radix Saposhnikoviae in rat plasma and urine.[Reference:
WebLink]
To analyze the constituents and metabolites of Radix Sa poshnikoviae (RS) in rat plasma and urine by rapid-resolution liquid chromatography-time of flight mass spectrometry (RRLC-TOF/MS), so as to explore the active ingredients and metabolites of RS in vivo.
METHODS AND RESULTS:
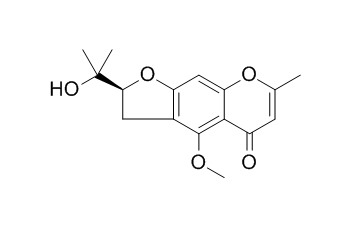
The separation was performed on a Angilent Zorbax Extend-C14 (5 μm, 250 mm x 4.6 mm id) column, with a methanol-water mobile phase system used for gradient elution. Time-of-flight mass spectrometer (TOF/MS) was applied for qualitative analysis under positive ion mode. Based on the accurate molecular weight of TOF/MS detection and the compound list of RS established previously, the constituents and metabolites of RS in different matrix in vivo were identified. Six constituents of RS were identified in the plasma, sucrose, prim-O-glucosylcimifugin, cimifugin, nodakenetin, 5-O-Methylvisamminol, and 3′-O-i-butyrylhammaudol. Eight constituents were identified in the urine, prim-O-glucosylcimifugin, divaricatacid, cimifugin, 4′-O-glucosyl-5-O- methylvisamminol, (3S)-2,2-dimethyl-3, 5-dihydroxy-8-hydroxymethyl-3, 4-dihydro-2H, 6H-benzo-[1, 2-b: 5, 4-b′] dipyran-6-one, 5-O-Methylvisamminol, see-O-β-D-glucosylhammaudol, and wogonin. Two metabolites were identified in the urine, glucuronide of cimifujin and an isomer of it.
CONCLUSIONS:
The present method is reliable and effective for identifying compounds of RS in vivo, and it can provide a reference and evidence for the further pharmacodynamics experiments.