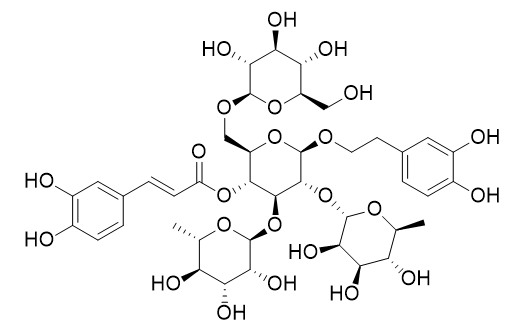
2'-Rhamnoechinacoside
2'-Rhamnoechinacoside is a natural product from Cistanche tubulosa.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmaceuticals (Basel).2021, 14(3):260.
Mol Med Rep.2022, 26(4):299.
J Biol Chem.2014, 289(3):1723-31
Molecules.2019, 24(24),4583
J of the Korean Society of Cosmetics and Cosmetology2019, 225-231
J Appl Pharm Sci.2022, 12(04):044-053
Microorganisms.2021, 9(12):2514.
J of Apicultural Research2020, 10.1080
Gene.2022, 815:146178.
J Biochem Mol Toxicol.2025, 39(8):e70416.
Related and Featured Products
Phytochemistry. 2015 Sep;117:185-93.
Phenylethanoid glycosides in tepals of Magnolia salicifolia and their occurrence in flowers of Magnoliaceae.[Pubmed:
26093323 ]
Phenylethanoid glycosides were among the major UV-absorbing components in 80% aq. CH3OH extracts of the tepals of Magnolia salicifolia (Siebold & Zucc.) Maxim. (Magnoliaceae; Magnolia subgenus Yulania).
METHODS AND RESULTS:
Structural characterisation of isolated compounds by spectroscopic and chemical methods revealed three previously unrecorded examples, yulanoside A, yulanoside B and 2'-Rhamnoechinacoside, and the known compounds echinacoside and crassifolioside; chromatographic methods also identified verbascoside in the tepal extract. Yulanoside A is the first reported example of a phenylethanoid pentaglycoside, namely hydroxytyrosol 1-O-{β-D-glucopyranosyl-(1→4)-β-D-glucopyranosyl-(1→6)-[3,4-dihydroxycinnamoyl-(→4)][α-L-rhamnopyranosyl-(1→3)][α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside}.
A survey of Magnolia sensu lato and Liriodendron (the two genera of Magnoliaceae) suggested that yulanoside A and its deglucosyl derivative (yulanoside B) were a feature of the tepal chemistry of Magnolia subgenus Yulania (except Magnolia acuminata, the sole member of section Tulipastrum, which did not accumulate phenylethanoid glycosides). The two species of Liriodendron and examined examples of Magnolia subgenus Magnolia sections Magnolia and Rytidospermum (subsection Oyama) also accumulated phenylethanoid glycosides in their tepals and in these species, and in subgenus Yulania, the major compounds were one or more of echinacoside, 2'-Rhamnoechinacoside, crassifolioside and verbascoside. Levels of phenylethanoid glycosides were found to be much lower in species studied from Magnolia sections Gwillimia, Macrophylla and Rytidospermum (subsection Rytidospermum), although yulanoside A was detectable in M. macrophylla and this may have some bearing on the placement of section Macrophylla, which is currently uncertain.
CONCLUSIONS:
In the isolates of yulanoside B and echinacoside, minor phenylethanoid glycosides were determined to be analogues of these compounds with β-D-xylose at C-3' of the primary glucose rather than α-L-rhamnose.