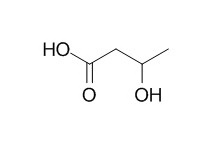
3-Hydroxybutyric acid
3-Hydroxybutyric acid is a ketone body and acts as an indicator of energy balance and a central regulator of energy homeostasis. 3-Hydroxybutyric acid has anesthetic actions, which are due to the metabolite's abilities to alter physical properties of cell membranes, leading to indirect effects on membrane protein function.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Psychopharmacology (Berl).2020, 10.1007
Molecules.2021, 26(9):2765.
Front Pharmacol.2021, 12:764297.
Int J Mol Sci.2022, 23(10):5468.
Int J Mol Med.2020, 45(5):1514-1524.
Mie University2019, 10076.
Cancer Manag Res.2019, 11:483-500
Chem Pharm Bull (Tokyo).2017, 65(9):826-832
Sci Rep.2019, 9(1):6429
Appl. Sci. 2021, 11(22), 10552
Related and Featured Products
Anal Biochem. 2012 Jun 15;425(2):114-6.
Enzymatic fluorometric microplate assay for quantitative analysis of 3-hydroxybutyric acid in mouse plasma.[Pubmed:
22449496]
3-Hydroxybutyric acid (3HB) is a ketone body and acts as an indicator of energy balance and a central regulator of energy homeostasis.
METHODS AND RESULTS:
We report the application of a sensitive fluorometric assay for the quantitative determination of 3HB. The assay is based on the oxidation of 3HB by 3HB dehydrogenase and on the diaphorase-resazurin amplifying system.
CONCLUSIONS:
This simple assay enables the measurement of changes in 3HB levels in the blood of normal mice by very small volume sample collection. Therefore, this assay will be useful for in vivo studies of small animals.
Langmuir. 2013 Feb 12;29(6):1948-55.
3-Hydroxybutyric acid interacts with lipid monolayers at concentrations that impair consciousness.[Pubmed:
23339286]
METHODS AND RESULTS:
3-Hydroxybutyric acid (also referred to as β-hydroxybutyric acid or BHB), a small molecule metabolite whose concentration is elevated in type I diabetes and diabetic coma, was found to modulate the properties of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) monolayers when added to the subphase at clinical concentrations. This is a key piece of evidence supporting the hypothesis that the anesthetic actions of BHB are due to the metabolite's abilities to alter physical properties of cell membranes, leading to indirect effects on membrane protein function. Pressure-area isotherms show that BHB changes the compressibility of the monolayer and decrease the size of the two-phase coexistence region. Epi-fluorescent microscopy further reveals that the reduction of the coexistence region is due to the significant reduction in morphology of the liquid condensed domains in the two-phase coexistence region.
CONCLUSIONS:
These changes in monolayer morphology are associated with the diminished interfacial viscosity of the monolayers (measured using an interfacial stress rheometer), which gives insight as to how changes in phase and structure may contribute to membrane function.