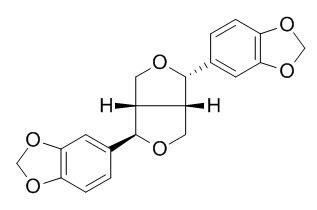
(-)-Asarinin
Asarinin, a mammalian lignan precursor, has immunosuppression activity in vitro. Asarinin can decrease peripheral blood concentration of interleukin (IL)-12 and inhibit the expression of Toll-like receptor 4 (TLR4) and chemokine (C-X-C motif) receptor 3 (CXCR3), which means asarinin may have a role on TLR4 pathway and produced prolongation of allograft heart survival.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plants (Basel).2023, 12(5):1120.
Tumour Biol.2015, 36(9):7027-34
Int J Mol Sci.2017, 19(1)
Nutrients.2018, 10(12):E1998
J Mass Spectrom.2022, 57(2):e4810.
Antioxidants (Basel).2020, 9(4):284.
SBRAS2016, 12
Biomedicines.2022, 10(2):463.
J Colloid Interface Sci.2022, 622:298-308.
BioResources J.2020, 15(3).
Related and Featured Products
Chinese Journal of Cardiology, 2003, 31(6):444-7.
Experimental study of the effects of Asarinin on immunosuppression activity in vitro.[Reference:
WebLink]
To test the extract of Asarum heterotropides called Asarinin in the immunosuppression activity in vitro and compared with cyclosporine A(CsA).
METHODS AND RESULTS:
Cardiomyocytes of adult wistar rats and spleen cells of adult SD rats were separated. 0.1 ml of the cardiomyocytes(2×10~(6)/ml) as stimulate cells and 0.1 ml of spleen cells(1×10~(7)/ml) as response cells were cultured in 96 culture plates. The model of rejection reaction in vitro was set up. Then the 0.1 ml serum including Asarinin was given to culture plates, the inhibition rate of lymphocyte transformation was investigated with MTT after 108 hours and IL-2、INF-#gamma#、IL-4 were detected with ELISA. Asarinin inhibited the lymphocyte transformation (inhibition rate 60.37%) in vitro.
CONCLUSIONS:
Compared with positive control group, it significantly decreased IL-2(69.11±17.47 pg/ml vs 160.44±19.79 pg/ ml, P<0.01)、INF-#gamma#(183.11± 95.24 pg/ml vs 521.89± 133.18 pg/ml, P<0.01) and increased IL-4(53.14±11.80 pg/ml vs 14.44± 4.21 pg/ml, P<0.01)in culture plates.
Transplant Proc. 2015 Mar;47(2):545-8.
The effect of Asarinin on Toll-like pathway in rats after cardiac allograft implantation.[Pubmed:
25769604]
The objective of this study was to study the mechanism of the anti-rejection effect of Asarinin in rats that underwent cardiac allograft implantation.
METHODS AND RESULTS:
Hearts from Wistar rats were transplanted into the abdominal cavity of Sprague Dawley rats (SD rats) 64 SD rats received either cyclosporin A (CsA), Asarinin, or demi-dose of cyclosporine A and Asarinin through oral administration. On the seventh day post-transplantation, the expression of Toll-like receptor 4 (TLR4), chemokine (C-X-C motif) receptor 3 (CXCR3) in myocardium, and the level of interleukin (IL)-12 in the peripheral blood were analyzed 7 days after transplantation.
The survival time in 3 groups (CsA group, Asarinin group, and semi-dose CsA group) prolonged (P < .01), the microscope myocardial histopathology in 3 groups (CsA group, Asarinin group and semi-dose CsA group) relieved, the expression of TLR4 and CXCR3 in 3 groups was significantly decreased (P < .01) when compared with the control group. The level of IL-12 decreased remarkably (P < .05) in the 3 groups when compared with the control group.
CONCLUSIONS:
The combined data suggested that Asarinin decreased peripheral blood concentration of IL-12 and inhibited the expression of TLR4 and CXCR3, which means Asarinin may have a role on TLR4 pathway and produced prolongation of allograft heart survival.
Phytochemistry, 1992, 31(3):757-60.
Inhibition of Δ5-desaturase in polyunsaturated fatty acid biosynthesis by (−)-asarinin and (−)-epiasarinin.[Reference:
WebLink]
METHODS AND RESULTS:
An extract of Chinese medicine, saishin (Asiasari radix), was found to increase the mycelial dihomo-γ-linolenic acid content of an arachidonic acid-producing fungus, Mortierelia alpina, with an accompanying decrease in its arachidonic acid content.
The factors responsible for this phenomenon were isolated and identified as (−)-asarinin and (−)-epiasarinin, which are the enantiomers of (+)-episesamin and (+)-sesamin, respectively. The inhibitory effects on the Δ5-desaturase in rat liver microsomes of these factors were in the order of (+)-sesamin > (−)-epiasarinin > (−)-asarinin > (+)-episesamin.
CONCLUSIONS:
Kinetic analysis showed that (−)-asarinin and (−)-epiasarinin are non-competitive inhibitors, the Kis for rat liver Δ5-desaturase being 2.8 × 10−4 and 7.1 × 10−4 M, respectively, which are almost the same as the values of the (+)-enantiomers.
Acta Crystallogr C. 2013 Jan;69(Pt 1):87-9.
Asarinin, C(20)H(18)O(6), was isolated as a racemate from the shrub Zanthoxylum alatum.
METHODS AND RESULTS:
Both forms of the enantiomerically pure substance, (+)- and (-)-Asarinin, have been the subject of a total of five previous structure determinations that are essentially identical except for the absolute stereochemistry. However, there seems to be some confusion in the literature concerning these structure determinations of asarinin and also those of its stereoisomer sesamin. The molecular structure of racemic asarinin differs from that of the pure enantiomers in the orientation of one ring system.
CONCLUSIONS:
In the packing of the racemate, molecules are linked by C-H...O interactions to form ribbons parallel to [101].
Food Chem., 2011, 124(3):895-9.
A new mammalian lignan precursor, asarinin.[Reference:
WebLink]
Enterolactone (ENL) and enterodiol are mammalian lignans.
METHODS AND RESULTS:
Several plant lignans have been reported as precursors of mammalian lignans. However, asarinin (AS), a furofuran type lignan which occurs in medicinal plants and foods, has not been reported as mammalian lignan precursor to date. After the incubation of AS with human intestinal microflora, AS was converted to not only ENL, but also two more metabolites (mono-demethylenated and ring-cleaved compounds). Under the same conditions, sesamin (SM) was converted to ENL. Furthermore, chiral HPLC analysis showed that ENL produced from AS and SM was (−)-ENL.
CONCLUSIONS:
This is the first report which shows that AS is a mammalian lignan precursor.